This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Principles – Effect of Galvanic Coupling”.
1. The effect of Galvanic Coupling is due to the contact of dissimilar metals in a conductive electrolyte.
a) True
b) False
View Answer
Explanation: If two dissimilar metals are placed in contact with each other in a conductive electrolyte, the potential difference produces electron flow between them. This results in the acceleration of preferential anode dissolution. This is known as the effect of Galvanic coupling.
2. What are the factors that affect Galvanic coupling?
a) Type of metals
b) Relative size of electrodes
c) Environmental conditions
d) Types of metals, the relative size of electrodes and environmental conditions
View Answer
Explanation: Metal combinations used for galvanic contact will affect based on the potential difference between them. Relative size of electrodes, environmental conditions such as temperature, humidity and salinity alter the chemical kinetics of the reaction.
3. Preferential cathode and anode are formed based on the potential value of a metal electrode.
a) True
b) False
View Answer
Explanation: Electrode with relatively high reduction potential preferentially act as a cathode and the other electrode will act as an anode. The effect of galvanic coupling can be minimized by selecting metal combinations with less potential difference.
4. Which of the following type of corrosion is depicted in the given figure?
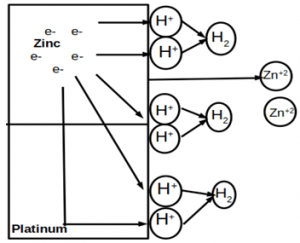
a) Uniform corrosion
b) Erosion corrosion
c) Galvanic corrosion
d) Crevice corrosion
View Answer
Explanation: Galvanic corrosion (dissimilar metal corrosion) is an electrochemical corrosion in which preferential anode will corrode rapidly due to the effect of galvanic coupling.
5. In which of the following cases, we observe the galvanic effect?
a) Bolts and Nuts metal joining’s
b) Piping arrangements
c) Machinery of a different metal combination
d) Bolts and nuts metal joining’s, piping arrangements and machinery of a different metal combinations
View Answer
Explanation: The galvanic effect is due to the contact of two dissimilar metals in a conductive solution. The corrosion caused due to galvanic effect is known as galvanic corrosion. It can be usually observed at bolts and nuts metal joining’s, piping arrangements and machinery of different metal combinations.
6. What is the abbreviation of SHE?
a) Substitute hydrogen electrode
b) Standard hydrogen electrode
c) Significant hydrogen electrode
d) Standard hydride electrode
View Answer
Explanation: SHE means a Standard hydrogen electrode. It is used to calculate the potential of other elements by arbitrarily defined as zero. The standard conditions of SHE is 1 atm pressure of H2 gas, 25°C temperature and non-corroding conditions for pure metals.
7. Which of the following are Reference electrodes?
a) Standard hydrogen electrode
b) Calomel electrode
c) Silver electrode
d) Standard hydrogen electrode and a calomel electrode
View Answer
Explanation: In EMF series all metals are referenced against the hydrogen electrode (H2/H+) which is arbitrarily defined as zero. This is known as the Standard hydrogen electrode (SHE). The saturated calomel electrode also acts as a reference electrode based on the reaction between elemental mercury (Hg) and mercury(I) chloride (Hg2Cl2). And the electrode potential value of the calomel electrode is +0.241 V.
8. Which of the following is/are the characteristics of a Galvanic series?
a) Used only for pure metals
b) Less accurate prediction than EMF series
c) Includes active and passive potentials of a metal
d) Measured only at room temperature
View Answer
Explanation: Galvanic series is the arrangement of various engineering materials (pure metals and alloys) in a decreasing reduction potential value in seawater. Galvanic series is more accurate than EMF series and it can be measured at various temperatures of a specific medium. It includes the active state potential and passive state potential of a metal.
9. Which of the following is/are the preventions of the galvanic effect?
a) Insulating dissimilar metals
b) Selection of close metal combinations in a galvanic series
c) Neglecting area effect
d) Insulating dissimilar metals and selection of close metal combinations in a galvanic series
View Answer
Explanation: The galvanic effect is due to the contact of two dissimilar metals in a conductive solution. It can be minimized by insulating dissimilar metals with non-conductive materials. And it can be reduced by the selection of close metal combinations in a galvanic series.
10. Which of the following effect is described in the given figure?
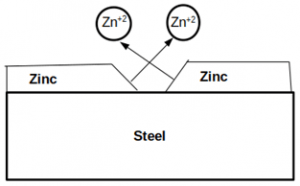
a) Crevice
b) Pitting
c) Beneficial galvanic effect
d) Erosion
View Answer
Explanation: The galvanic effect is due to the contact of two dissimilar metals in a conductive solution. Here beneficial galvanic effect takes place as zinc is corroding preferentially by protecting steel.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mechanical Engineering Books
- Apply for Mechanical Engineering Internship
- Apply for Metallurgical Engineering Internship
- Check Metallurgical Engineering Books
- Practice Mechanical Engineering MCQs
