This set of Basic Corrosion Engineering Questions and Answers focuses on “Environments – Sea Water and Fresh Water”.
1. Which of the following is/are true regarding seawater?
a) It contains 3.4% salt
b) It has a pH around 8
c) It is a good electrolyte with high electrical conductivity
d) Seawater is a good electrolyte with high electrical conductivity with 3.4% salt and pH around 8
View Answer
Explanation: Seawater is a good electrolyte with high electrical conductivity with 3.4% salt and pH around 8. It can cause galvanic and crevice corrosion to various metals.
2. Which of the following is/are the factors that affect corrosion in seawater?
a) Temperature and velocity
b) Oxygen content and biological organisms
c) Temperature, velocity, oxygen content and biological organisms
d) Temperature, velocity and oxygen content
View Answer
Explanation: The factors that affect the corrosion of materials in seawater are:
i. Temperature
ii. Velocity
iii. Oxygen content
iv. Biological organisms.
3. Which of the following region of the seacoast environment have high corrosion based on the given figure?
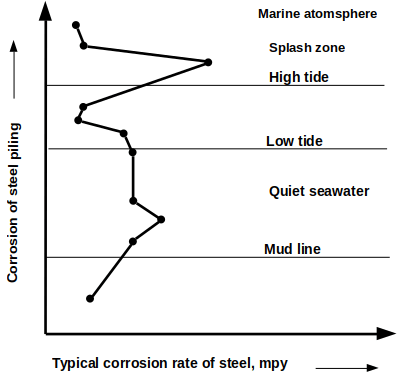
a) Quiet seawater
b) Splash region
c) High tide area
d) Mud line
View Answer
Explanation: Greatest corrosion occurs in the splash region due to alternate wetting and drying and also aeration. We know oxygen and moisture are the primary requirements for corrosion of metals.
4. Which of the following regions of the seacoast environment shows the effect of the pitting of metals and alloys?
a) Quiet seawater
b) High tide region
c) Low tide region
d) Splash region
View Answer
Explanation: Pitting is extremely localized corrosion which results in holes or cavities. Quiet seawater in the region of the seacoast environment that shows the effect of the pitting of metals and alloys. An increase in the velocity of a corrosive solution decreases the pitting tendency of a metal.
5. Corrosion by seawater at greater depth is usually decreased because of the lower temperature.
a) False
b) True
View Answer
Explanation: Corrosion by seawater at greater depth is usually decreased because of the lower temperature. It decreases by 40°F for one-mile depth.
6. Which of the following impurities contaminates the brackish water due to tidal action in rivers and bays near the ocean?
a) Chlorides
b) Bromides
c) Hydrides
d) Metal oxides
View Answer
Explanation: Chloride ions are the impurities that contaminate the brackish water due to the tidal action in rivers and bays near the ocean. The source of these chloride ions is sodium chloride in seawater.
7. Which of the following metals have high resistance to crevices in quiet seawater?
a) Hastelloy C
b) Titanium
c) Hastelloy C and Titanium
d) Nickel-copper alloy
View Answer
Explanation: Hastelloy C and titanium have high resistance to crevices in quiet seawater. Due to this, these metals are used for various corrosive seawater applications. Whereas nickel-copper alloy shows less resistance.
8. Which of the following alloy has high cavitation resistance that usually used for ship propellers and pumps impellers in seawater?
a) Titanium
b) Stellite
c) 17-7 Stainless steel
d) Nickel aluminum bronze
View Answer
Explanation: Stellite is a cobalt-chromium alloy with high cavitation resistance that usually used for ship propellers and pump impellers in seawater. Composition of Stellite is 27–32% chromium, 4–6% tungsten, 0.9–1.4% carbon, with additions of nickel, iron, silicon, manganese, and molybdenum and cobalt as balance.
9. Which of the following factors that affect the corrosivity in freshwater?
a) Oxygen content
b) Hardness
c) Chloride content
d) Oxygen content, hardness and chloride content
View Answer
Explanation: Factors that affect the corrosivity in freshwater are:
i. Oxygen content
ii. Hardness
iii. Chloride and sulfur content.
10. Which of the following type of water is more corrosive?
a) Hard water
b) Hard and soft water
c) Soft water
d) High-purity water
View Answer
Explanation: In hard water, carbonates often deposit on the metal surface and protect it from corrosion. Whereas in soft water protective deposits do not form. Hence, soft water is more corrosive than hard water and high-purity water.
11. Which of the following metals are widely used for handing freshwater?
a) Cast iron
b) Stainless steel
c) Galvanized steel
d) Cast iron, stainless steel, and galvanized steel
View Answer
Explanation: Cast iron, stainless steel, steel, and galvanized steel are widely used for handling freshwater. Metals such as copper, brass, aluminum, monel, and cupronickels are also used with the factors of temperature, contamination, and longer life.
12. Which of the following corrosion defects that affect the reliability of tubing?
a) Selective weld metal attack
b) Improper pickling and heat treatment
c) Residual stresses, selective weld metal attack, improper pickling, and heat treatment
d) Selective weld metal attack and residual stresses
View Answer
Explanation: Corrosion defects that affect the reliability of tubing are:
i. Inadequate alloying
ii. Selective weld metal attack
iii. Improper pickling
iv. Residual stresses
v. Improper heat treatment.
13. Corrosion decreases with an increase in the purity of water.
a) True
b) False
View Answer
Explanation: Corrosion decreases with increasing purity of the water because of less solid and gases and increasing electrical resistance. Resistance is a measure of water purity.
14. Which of the following types of corrosion has been seen in stainless steels in high purity water containing oxygen?
a) Cracking of solution-quenched stainless steel only
b) Intergranular attack and cracking of solution-quenched steel
c) Intergranular attack only
d) Neither intergranular corrosion nor cracking of solution-quenched steel
View Answer
Explanation: Intergranular corrosion and cracking of solution-quenched stainless steel and alloys have been observed in high-purity water containing oxygen.
15. Which of the following metals/alloys are used for handling high-purity water in nuclear applications?
a) Zirconium and its alloys
b) Stainless steels
c) Inconel and Incoloy
d) Zirconium and its alloys, stainless steels, Inconel and Incoloy
View Answer
Explanation: Zirconium and its alloys, stainless steels, Inconel and Incoloy are the metals/alloys that are used for handling high-purity water in nuclear applications.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice basic questions and answers on all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Metallurgical Engineering Books
- Apply for Metallurgical Engineering Internship
- Apply for Mechanical Engineering Internship
- Check Mechanical Engineering Books
- Practice Mechanical Engineering MCQs
