This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “High-Temperature Corrosion – 1”.
1. Which of the following is/are the systems that are prone to high-temperature corrosion?
a) Gas turbines
b) Rocket engines
c) Furnaces
d) Gas turbines, rocket engines, and furnaces
View Answer
Explanation: High-temperature systems such as gas turbines, rocket engines, and furnaces are prone to high-temperature corrosion due to high working temperature and petrochemical corrosive atmosphere around it.
2. Pilling and Bed worth proposed that oxidation resistance is related to the volume ratio of oxide and metal per grams atom of metal.
a) True
b) False
View Answer
Explanation: Pilling and Bed worth proposed that oxidation resistance of a metal oxide is related to the volume ratio of oxide and metal per grams atom of metal.
3. Which of the following is/are the characteristics of a metal oxide if the Pilling and Bed worth ratio is less than 1?
a) Protective
b) Unprotective
c) Unprotective and insufficient oxide
d) Unprotective and sufficient oxide
View Answer
Explanation: If Pilling and Bed worth ratio of a metal oxide is less than 1 then it is an unprotective and insufficient oxide, if it is near to 1 then it is protective and adherent and if it is greater than 1 then it is unprotective.
4. Which of the following is/are the causes for the poor oxidation resistance of metal oxide with high Pilling and Bed worth ratio?
a) Large compressive stresses in the oxide
b) Cracking
c) Spalling
d) Large compressive stresses in the oxide, cracking and spalling
View Answer
Explanation: Metal oxides with a value greater than 1 Pilling and Bed worth ratio have poor oxidation resistance due to large compressive stresses in the oxide, cracking, and spalling.
5. Which of the following is/are the ideal characteristics of the passive metal oxide layer?
a) Good adherence and high melting point
b) Low electrical conductivity and low vapor pressure
c) Good adherence, high melting point, low vapor pressure, and low electrical conductivity
d) Good adherence and high vapor pressure
View Answer
Explanation: Ideal characteristics of passive metal oxides layer are:
- Good adherence
- High melting point
- Low electrical conductivity
- Low vapor pressure
- Low diffusion coefficient.
6. Which of the following metal has a nonprotective oxide?
a) Cadmium (CdO2)
b) Copper (Cu2O)
c) Chromium (Cr2O3)
d) Silicon (SiO2)
View Answer
Explanation: Metal oxides can be categorized into protective and nonprotective based on the value of the Pilling and Bed worth ratio. Here, copper (1.68), chromium (1.99), and silicon (2.27) are protective in nature whereas oxide of cadmium (1.21) is unprotective.
7. What is the value of the Pilling and Bed worth ratio of tungsten?
a) 1.21
b) 3.40
c) 2.60
d) 0.45
View Answer
Explanation: Pilling and Bed worth ratio is defined as the volume ratio of oxide and metal per grams atom of metal. The Pilling and Bed worth ratio of tungsten is around 3.40 and it is unprotective in nature.
8. Which of the following is/are the interface at which new metallic oxides (MO) are produced?
a) Metal-scale interface
b) Scale-gas interface
c) Metal-scale interface or scale-gas interface
d) Neither metal-scale interface nor scale-gas interface
View Answer
Explanation: New metallic oxides (MO) is produced at a metal-gas interface or scale-gas interface based on the diffusion coefficient of metallic and oxygen ions in the metal matrix.
9. The diffusion of either cation or oxygen ions usually controls the reaction rate of high-temperature metallic oxidation.
a) True
b) False
View Answer
Explanation: High-temperature metal-oxygen interaction can’t be separated electrically whereas diffusion of either cation or oxygen ions usually controls the reaction rate of high-temperature metallic oxidation.
10. Which of the following is depicted in the given figure?
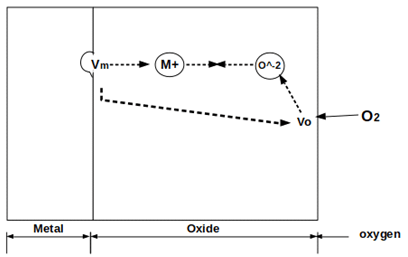
a) Metal-liquid oxidation
b) Metal-gas oxidation
c) Metal-metal oxidation
d) Aqueous metal corrosion
View Answer
Explanation: The given figure depicts the metal-gas oxidation and it also depicted the electrochemical process occurring during gaseous oxidation. It includes metal, metal oxide, and an oxygen atmosphere.
11. Which of the following order is correct regarding the oxides of iron starting from metallic iron to gaseous oxygen interface?
a) FeO, Fe2O3, and Fe3O4
b) Fe2O3, Fe3O4 and FeO
c) Fe3O4, Fe2O3, and FeO
d) FeO, Fe3O4, and Fe2O3
View Answer
Explanation: The most oxygen-rich compound is found at the scale-gas interface whereas a metal-rich compound is found at the metal-scale interface. Therefore, the correct order is FeO(22.270% O2), Fe3O4(27.641% O2), and Fe3O4(30.057% O2).
12. The relative thickness of each phase of a metallic oxide is determined by the rate of ionic diffusion through that phase.
a) True
b) False
View Answer
Explanation: The relative thickness of each phase of a metallic oxide is determined by the rate of ionic diffusion through that phase. Diffusion of ions may include metallic ions such as Fe+2 or Fe+3 or oxygen ions.
13. Which of the following metal(s) produces metal oxide at a metal-scale interface?
a) Titanium
b) Niobium
c) Zirconium
d) Titanium, Niobium, and Zirconium
View Answer
Explanation: Titanium, Niobium, Zirconium, Hafnium, and Tantalum are the metals that produce metal oxides at the metal-scale interface. It is due to the predominant diffusion of oxygen-ion rather than metallic cation.
14. Which of the following is/are the n-type semiconducting oxides?
a) CdO2
b) TiO2
c) Al2O3, CdO2, and TiO2
d) CoO
View Answer
Explanation: Semiconducting oxides such as CdO2, Al2O3, CdO, TiO2, Ta2O5, and SiO2 are n-type semiconductors. It contains an excess of negatively charged electronic current carriers I.e. electrons.
15. Which of the following is/are the p-type semiconducting oxides?
a) FeO and MnO
b) FeO, MnO, Cu2O, and CoO
c) Al2O3, MnO, and CoO
d) CdO2, Cu2O, and Mno
View Answer
Explanation: Semiconducting oxides such as FeO, MnO, Cu2O, and CoO are the p-type semiconducting oxides whereas Al2O3, CdO2 are n-type oxides. In this, ionic transport occurs by metallic vacancies.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Metallurgical Engineering MCQs
- Check Mechanical Engineering Books
- Apply for Metallurgical Engineering Internship
- Apply for Mechanical Engineering Internship
- Practice Mechanical Engineering MCQs
