This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Prevention – Material Selection and Design – 1”.
1. The most common method of preventing corrosion is the selection of the proper metal or alloy for a particular corrosive service.
a) True
b) False
View Answer
Explanation: There is no such single material that can be used in all environment conditions and all temperature ranges. So, it is important to select a proper metal or alloy for a particular corrosive service. It prevents or reduces the corrosion damage of the metallic structure.
2. Which of the following composition range is correct regarding stainless steel?
a) 11.5 to 30% chromium and 10 to 22% nickel
b) 11.5 to 30% chromium and 0 to 22% nickel
c) 0 to 30% chromium and 0 to 22% nickel
d) 0 to 30% chromium and 10 to 22% nickel
View Answer
Explanation: Stainless steel is the generic name for a series of more than 30 different alloys with composition ranges from 11.5% to 30% chromium and 0 to 22% nickel and other alloying elements.
3. Which of the following types of corrosion in which stainless steels are more susceptible than ordinary steels?
a) Pitting corrosion
b) Stress-corrosion cracking
c) Intergranular corrosion
d) Stress-corrosion cracking, pitting and intergranular corrosion
View Answer
Explanation: Stainless steels are more susceptible to localized corrosion such as intergranular corrosion, stress-cracking corrosion, and pitting corrosion than ordinary steels. Instability of oxide layer in severe corrosive conditions and sensitizing temperature are the reasons for the susceptibility of stainless steel.
4. Which of the following metal is the best material selection combination for the nitric acid environment?
a) Tin
b) Nickel and its alloys
c) Stainless steels
d) Steel
View Answer
Explanation: Stainless steels are the best combination of metal for the nitric acid environment. Stainless steels readily form a passive oxide film in nitric acid. This minimizes or reduces the corrosion damage.
5. Which of the following metal is the best material selection combination for the caustic environment?
a) Titanium
b) Nickel and its alloys
c) Steels
d) Aluminum and its alloys
View Answer
Explanation: Nickel and its alloys are the best combination of metal for the caustic environment. Metals such as titanium and high tensile strength steels are prone to caustic embrittlement.
6. Which of the following metal is the best material selection combination for distilled water?
a) Tin
b) Lead
c) Titanium
d) Nickel
View Answer
Explanation: Tin is the best combination of metal for distilled water. Tin or tin coatings are almost always chosen as a container or piping material for very pure distilled water.
7. Which of the following metal is/are the correct combination for reducing or non-oxidizing environment?
a) Nickel and its alloys
b) Copper and its alloys
c) Stainless steels
d) Nickel, copper and its alloys
View Answer
Explanation: Nickel, copper and its alloys are the correct combinations of metals for reducing or non-oxidizing environments. Whereas stainless steels and titanium alloys are stable in an oxidizing environment.
8. Which of the following material is used for handling hydrogen peroxide?
a) Commercially pure metals
b) Alloys
c) Both commercially pure metal and alloys
d) Neither commercially pure metals nor alloys
View Answer
Explanation: Commercially pure metal such as aluminum (99.5+ purity) is used for handling hydrogen peroxide. Alloys result in the decomposition of hydrogen peroxide due to the catalytic action of alloying elements.
9. Which of the following type of zirconium is used in an atomic-energy application?
a) Induction-melted zirconium
b) Arc-melted zirconium
c) Both arc-melted and induction-melted zirconium
d) Neither arc-melted nor induction melted zirconium
View Answer
Explanation: Arc-melted zirconium is more corrosion resistant than induction-melted zirconium because of more impurities in the latter. Hence arc-melted zirconium is used in an atomic-energy application.
10. Which of the following is/are the different classes of non-metallics?
a) Ceramics and wood
b) Plastics and ceramics
c) Wood, ceramics, and plastic
d) Ceramics only
View Answer
Explanation: The five general classes of non-metallics are:
11. Which of the following type of materials possess excellent corrosion and high-temperature resistance?
a) Metals
b) Plastics
c) Ceramics
d) Both ceramics and metals
View Answer
Explanation: Ceramics are the class of materials that possess excellent corrosion and high-temperature resistance. But brittleness or less tensile strength is the primary limitation of ceramics.
12. Boiling seawater is less corrosive than that of hot seawater.
a) True
b) False
View Answer
Explanation: Boiling seawater is less corrosive than that of hot seawater. Because the solubility of oxygen in seawater decreases with an increase in temperature. Reduction of oxygen results in severe metallic dissolution in many oxygenated corrosive solutions.
13. Which of the following belongs to the alteration of the environment to minimize corrosion?
a) Decreasing velocity
b) Removing oxygen and oxidizers
c) Changing concentration
d) Decreasing velocity, removing oxygen and oxidizers and changing the concentration
View Answer
Explanation: Alteration of the environment is one of the measures to minimize corrosion. Alteration of the environment includes
14. Which of the following is/are the functions of magic devices or water-conditioning gadgets?
a) Prevent scaling
b) Destroy bacteria
c) Reduce water hardness
d) Prevent scaling, destroy bacteria and reduce water hardness
View Answer
Explanation: Magic device or water-conditioning gadgets are the devices to control water corrosion. Purpose of these gadgets are
15. Which of the following metal combination is/are selected as per given requirement in the figure?
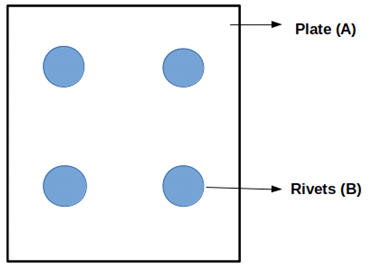
a) Gold – Iron
b) Steel – Stainless
c) Brass – Copper
d) Silver – Zinc
View Answer
Explanation: The given figure indicates the galvanic metal combination. Hence metal combination should be as close as possible in the galvanic series to minimize the galvanic effect. Hence good metal combination is brass and copper.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Check Corrosion Engineering Books
- Practice Metallurgical Engineering MCQs
- Practice Mechanical Engineering MCQs
- Apply for Metallurgical Engineering Internship
