This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Eight Forms – Hydrogen Damage”.
1. Hydrogen damage refers to the mechanical damage of a metal caused by the presence of or interaction of hydrogen.
a) True
b) False
View Answer
Explanation: Hydrogen damage refers to the mechanical damage of a metal caused by the presence of or interaction of hydrogen. And hydrogen damage can be classified into four types based on the mechanism of failure.
2. Which of the following is/are the types of hydrogen damage?
a) Hydrogen blistering
b) Hydrogen embrittlement
c) Decarburization
d) Hydrogen blistering, hydrogen embrittlement, and decarburization
View Answer
Explanation: Classification of hydrogen damage:
- Hydrogen blistering
- Hydrogen embrittlement
- Decarburization
- Hydrogen attack.
3. What is meant by hydrogen blistering?
a) Entrapment of hydrogen molecules in the metal voids
b) Formation of brittle metal hydrides
c) Entrapment of metal hydrides in the metal voids
d) Entrapment of hydrogen molecules and metal hydrides in the metal voids
View Answer
Explanation: Hydrogen blistering refers to the local deformation of a metal due to the entrapment of hydrogen molecules in the metal voids. Initially, hydrogen atoms diffuse into metal and react with each other to form a hydrogen molecule.
4. What is meant by hydrogen embrittlement?
a) Entrapment of hydrogen molecules in the metal voids
b) Formation of brittle metal hydrides
c) Entrapment of metal hydrides in the metal voids
d) Entrapment of hydrogen molecules and metal hydrides in the metal voids
View Answer
Explanation: Hydrogen embrittlement refers to the loss of ductility and strength of a metal due to the formation of brittle metal hydrides. Strong hydride forming metals such as titanium, results in brittle fracture.
5. Which of the following types of hydrogen damage processes are high-temperature processes?
a) Hydrogen blistering and hydrogen attack
b) Hydrogen attack and Decarburization
c) Decarburization and hydrogen embrittlement
d) Hydrogen attack only
View Answer
Explanation: Hydrogen attack and decarburization are high-temperature processes. Decarburization refers to the removal of carbon from metal, is often produced by moist hydrogen at high temperatures. Hydrogen attack refers to the interaction of hydrogen and metal at high temperatures.
6. Which of the following industries has a major concern on hydrogen blistering?
a) Petroleum industry
b) Chemical manufacturing industries
c) Oil and natural gas refineries
d) Oil and natural gas refineries, petroleum and chemical manufacturing industries
View Answer
Explanation: Hydrogen blistering refers to the local deformation of a metal due to the entrapment of hydrogen molecules in the metal voids.
Industries that have a major concern on hydrogen blistering are:
- Petroleum industry
- Chemical manufacturing industries
- Oil and natural gas refineries
- Welding workshops.
7. Which of the following type of compounds act as an inhibitor for hydrogen-ion reduction?
a) Sulfide ions
b) Phosphorous compounds
c) Arsenic compounds
d) Sulfide ions, phosphorous and arsenic compounds
View Answer
Explanation: Hydrogen atoms diffuse into steel and other metals, which results in hydrogen blistering and hydrogen embrittlement. These inhibitors such as sulfide ions (polysulfide ion), phosphorous, and arsenic compounds reduce the rate of hydrogen-ion reduction.
8. Which of the following type of corrosion is depicted in the given figure?
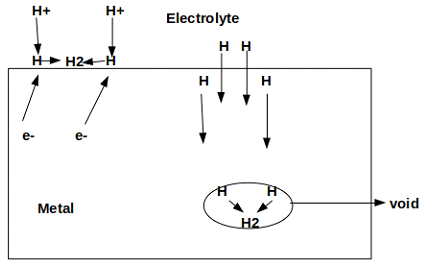
a) Filiform corrosion
b) Hydrogen embrittlement
c) Hydrogen blistering
d) Uniform corrosion
View Answer
Explanation: Hydrogen blistering is depicted in the given figure. It refers to the local deformation of metal with the entrapment of hydrogen molecules in the metal voids.
9. Which of the following type of corrosion is depicted in the given figure?
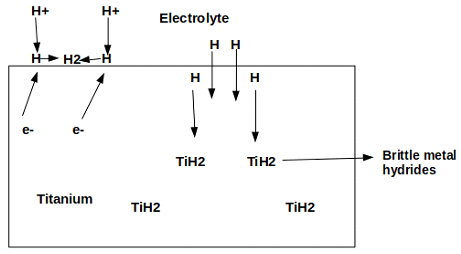
a) Hydrogen blistering
b) Hydrogen embrittlement
c) Filiform corrosion
d) Selective leaching
View Answer
Explanation: Hydrogen embrittlement is depicted in the given figure. It refers to the loss of ductility and tensile strength of metal due to the formation of the respective metal hydride.
10. Which of the following metal is highly susceptible to hydrogen embrittlement?
a) Titanium
b) Nickel
c) Martensitic iron-base alloys
d) Titanium and martensitic iron-base alloys
View Answer
Explanation: Hydrogen embrittlement refers to the brittle cracking failure of reactive metals due to the formation of metal hydrides. Titanium, ferritic, and martensitic iron-base alloys are susceptible to hydrogen embrittlement.
11. Which of the following type of corrosion in which corrosion occurs due to the presence of hydrogen sulfide?
a) Hydrogen embrittlement
b) Hydrogen blistering
c) Sulfide stress corrosion
d) Erosion corrosion
View Answer
Explanation: Sulfide stress cracking occurs in the presence of water and hydrogen sulfide. It is highly concerned with petroleum industries and oil refining industries.
12. Which of the preventions is/are the preventions of hydrogen blistering?
a) Use of clean steel instead of rimmed steel
b) Applying metallic, inorganic and organic coatings
c) Use of substituting alloys such as nickel-based alloys
d) Use of clean steel instead of rimmed steel, applying metallic, inorganic and organic coatings and use of nickel-based alloys
View Answer
Explanation: Preventions of hydrogen blistering are:
- Use of clean steel instead of rimmed steel
- Applying metallic, inorganic and organic coatings
- Use of substituting alloys such as nickel-based alloys
- Removing poisons such as sulfides, arsenic compounds, cyanides, and phosphorus ions.
13. Which of the following is/are the preventions of hydrogen embrittlement?
a) Baking of steels at low temperatures (200°F–300°F)
b) Alloying with nickel or molybdenum reduces susceptibility
c) Practicing proper welding with low hydrogen welding rods
d) Baking of steels at low temperatures, alloying with nickel or molybdenum reduces susceptibility and practicing proper welding with low hydrogen welding rods.
View Answer
Explanation: Preventions of hydrogen embrittlement are:
- Baking of steels at low temperatures (200°F–300°F)
- Alloying with nickel and molybdenum reduces susceptibility
- Practicing proper welding with low hydrogen welding rods.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mechanical Engineering Books
- Check Corrosion Engineering Books
- Apply for Mechanical Engineering Internship
- Check Metallurgical Engineering Books
- Practice Metallurgical Engineering MCQs
