This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Eight Forms – Pitting Corrosion”.
1. Pitting is a form of extremely localized attack that results in holes in the metal.
a) True
b) False
View Answer
Explanation: Pitting and crevice corrosion are the localized corrosion types, that results in the corrosion on very confined corrosive sites. Localized attack of pitting results in holes in the metal.
2. Which of the following is the characteristic of a pit?
a) Cavity with the surface diameter about the less than the depth
b) Cavity with the surface diameter about the same as or less than the depth
c) Cavity with the surface diameter about the same as or high than the depth
d) Cavity with the surface diameter about the high than the depth
View Answer
Explanation: Pitting is extremely localized corrosion that results in pits or cavity. A pit is a cavity with the surface diameter about the same as or less than the depth.
3. Which of the following form of corrosion is more destructive and insidious in nature?
a) Uniform corrosion
b) Intergranular corrosion
c) Pitting corrosion
d) Galvanic corrosion
View Answer
Explanation: Pitting is one of the most destructive and insidious forms of corrosion. It causes equipment to fail because of perforation with only a small percent weight loss of the entire structure.
4. Which of the following are the reasons that make it difficult to detect pits?
a) Small size
b) Varying depths
c) Pits covered with corrosion products
d) Small size, varying depths and covered with corrosion products
View Answer
Explanation: It is difficult to measure the pitting quantitatively because of its small size, varying depth, and due to covered corrosion products. It fails the equipment because of perforation over the entire surface with only a small percent weight loss.
5. Which of the following metal is highly prone to pitting corrosion?
a) 18-8 stainless steel by sulfuric acid with FeCl3
b) Titanium by sulfuric acid with FeCl3
c) Nickel by sulfuric acid with FeCl3
d) Copper by sulfuric acid with FeCl3
View Answer
Explanation: Pitting is one of the most destructive and insidious forms of corrosion. 18-8 stainless steel is highly prone to pitting corrosion by sulfuric acid in the presence of FeCl3. Whereas other metals are relatively resistant to pitting. Because it mainly depends on oxide film for its corrosion-resistant.
6. What is the usual direction for the growth of pits?
a) Vertically downwards
b) Vertically upwards
c) Horizontally sideward
d) Any primary direction
View Answer
Explanation: Pits usually grow in the direction of gravity i.e. vertically downwards. Gravitational force holds the corrosive solution vertically downwards in a cavity, that makes the pit growth downwards. And very a smaller number of pits grown vertically upwards.
7. Pitting usually requires an incubation period ranges from months to years.
a) False
b) True
View Answer
Explanation: Pitting can occur in two steps: i. Pit initiation ii. Pit growth.
The incubation period is the time required for a system to initiate pits on the metal surface. It usually ranges from months to years.
8. Which of the following corrosion form is depicted in the given figure?
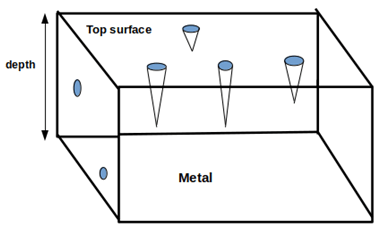
a) Crevice corrosion
b) Uniform corrosion
c) Intergranular corrosion
d) Pitting corrosion
View Answer
Explanation: Here, pitting corrosion is depicted in the figure. Pitting is extremely localized corrosion that results in pits or cavity and it is the most destructive and insidious form of corrosion.
9. Which of the following corrosion form is considered as the intermediate stage between uniform corrosion and complete corrosion resistance?
a) Galvanic corrosion
b) Pitting corrosion
c) Intergranular corrosion
d) Erosion corrosion
View Answer
Explanation:
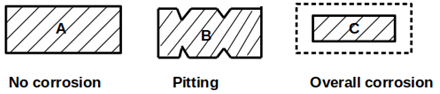
Case A: No corrosion of metal
Case B: Intense pitting on specific points
Case C: Uniform corrosion
10. Which of the following corrosion form is/are autocatalytic in nature?
a) Pitting and crevice corrosion
b) Crevice corrosion only
c) Pitting corrosion only
d) Pitting and intergranular corrosion
View Answer
Explanation: Autocatalytic is a phenomenon of rapid corrosion that occurs within the pit or crevice, while oxygen reduction takes place on adjacent surfaces. This process is self-stimulating and self-propagating. It occurs in both pitting and crevice corrosion.
11. Which of the following statement is correct regarding pitting corrosion?
a) Adjacent surfaces cathodically protect pits
b) Pits cathodically protect adjacent surfaces
c) Neither pits nor adjacent surfaces protect each other
d) Both pits and adjacent surfaces protect each other
View Answer
Explanation: Pits cathodically protect adjacent surfaces of metal; this is also called as cathodic protection. This is due to the autocatalytic nature of pitting corrosion. Hence, pits cathodically protect adjacent surfaces is the correct statement.
12. Which of the following is/are the difference between the crevice and pitting corrosion?
a) Autocatalytic nature
b) Corrosion mechanism
c) Initiation method
d) Both the corrosion mechanism and initiation method
View Answer
Explanation: Pitting is a special case of crevice corrosion. But in the view of the initiation method, these two-corrosion processes are different. Pitting corrosion is a self-initiating corrosion form, whereas crevice corrosion needs the formation of crevice (a small gap) with differential concentration from outside.
13. Which of the following ions have a high tendency to pitting corrosion?
a) Chlorides
b) Bromides
c) Hypo chlorites
d) Chlorides, bromides, and hypochlorites
View Answer
Explanation: In practical applications, most pitting failures are caused by chloride ions, hypochlorites, and bromide ions. Oxidizing metal ions with chlorides are aggressive pitters. Cupric, ferric, and mercuric halides are extremely aggressive.
14. Which of the following ions, that can reduce pitting corrosion?
a) Hydroxide salts
b) Chromate salts
c) Silicate salts
d) Hydroxide, chromate and silicate salts
View Answer
Explanation: Pitting is one of the most destructive and insidious forms of corrosion. The tendency of pitting can be increased with the presence of chloride, bromide, and hypochlorites. But this tendency can be prevented or minimized with the presence of hydroxide, chromate, and silicate salts.
15. Which of the following corrosion test is most reliable to know the extent of pitting corrosion?
a) To measure the average depth of pits
b) To measure the maximum depth of a pit
c) Weight loss method
d) Weight gain method
View Answer
Explanation: The measurement of maximum depth of pit would be a more reliable way of expressing pitting corrosion. As metal loss or metal gain (corrosion product) is very small and does not indicate the depth of penetration.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Metallurgical Engineering Books
- Check Mechanical Engineering Books
- Check Corrosion Engineering Books
- Apply for Mechanical Engineering Internship
- Apply for Metallurgical Engineering Internship
