This set of Corrosion Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Cathodic and Anodic Protection – 1”.
1. Which of the following is/are the common electrochemical reactions that occur during corrosion?
a) M==>M++ne
b) 2H++2e=>H2
c) O2+4H++4e==>H2O
d) M==>M++ne, 2H++2e=>H2 and O2+4H++4e==>H2O
View Answer
Explanation: The common electrochemical reactions that occur during corrosion are:
2. Cathodic protection is achieved by supplying electrons to the metal structure to be protected.
a) True
b) False
View Answer
Explanation: Cathodic protection is achieved by supplying electrons to the metal structure to be protected. It can be done by using impressed current in the range of microamperes per unit area of the metallic structure.
3. Which of the following cases in which the metal structure is protected?
a) Current passes from metal to electrolyte
b) Current enters it from the electrolyte
c) Electrical contact with dissimilar metals
d) Connecting the positive terminal of the power supply
View Answer
Explanation: Metallic structure can be protected when the impressed current is arranged to enter it from the electrolyte. Whereas current passes from metal to electrolyte, electrical contact with dissimilar metals, and connecting the positive terminal of the power supply results in accelerated corrosion.
4. Which of the following is/are the types of cathodic protection?
a) External impressed current supply
b) Sacrificial anode
c) External impressed current supply and sacrificial anode
d) Neither external impressed current supply nor sacrificial anode
View Answer
Explanation: Cathodic protection is achieved by supplying electrons to the metal structure to be protected. Types of cathodic protection are i. External impressed current supply ii. Sacrificial anode.
5. Which of the following terminal of the power supply should be connected to the metal to be protected?
a) Negative terminal
b) Positive terminal
c) Both positive and negative terminals
d) Either positive or negative terminal
View Answer
Explanation: As the conventional electrical theory states that current flows from (+) to (-), the structure is protected if the negative terminal of the power supply is connected metallic structure.
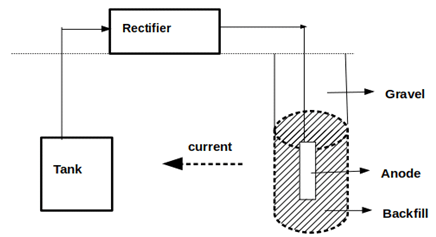
6. Which of the following method is depicted in the given figure?
a) Anodic protection
b) Impressed current supply
c) Sacrificial anode
d) Anodic and cathodic protection
View Answer
Explanation: Impressed current supply is a method of cathodic protection that is achieved by supplying electrons to the metal structure to be protected. The given figure depicted is impressed current supply method of an underground tank.
7. Which of the following material is/are used as an impressed-currents anode?
a) Graphite
b) Platinized titanium
c) Silicon-iron
d) Graphite, platinized titanium, and silicon-iron
View Answer
Explanation: Materials that can be used as an impressed-currents anode are:
8. Which of the following materials is/are used as backfill around the anode in impressed currents?
a) Coke breeze
b) Gypsum
c) Bentonite
d) Coke breeze, gypsum, and bentonite
View Answer
Explanation: Coke breeze, gypsum, and bentonite are the materials that are used as backfill material around the anode. These materials improve the electric contact between the anode and surrounding soil.
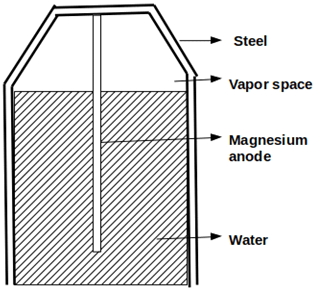
9. Which of the following protection method is depicted in the given figure?
a) Impressed-current supply method
b) Anodic protection
c) Sacrificial anode
d) Anodic protection and sacrificial anode
View Answer
Explanation: Sacrificial anode method is one the type of cathodic protection in which a metal (anode) corrodes preferentially than the metallic structure. Sacrificial anodes are inexpensive and easily available in nature.
10. Which of the metallic structure will require more impressed current density for cathodic protection?
a) Water heaters in hot freshwater
b) Underground pipelines
c) The storage tank of H2SO4
d) Reinforcement rods
View Answer
Explanation: Impressed method current is an external supply of power to provide electrons to metallic structure that needs to be protected. The storage tank of H2SO4 requires the highest current density per sq. feet due to the high corrosiveness of acid.
11. Which of the following reference electrode is used for cathodic-protection surveys?
a) Standard hydrogen electrode
b) Copper/copper sulfate reference electrode
c) Calomel electrode
d) Either calomel or standard hydrogen electrode
View Answer
Explanation: Copper/copper sulfate reference electrode is used for cathodic protection surveys. The potential of a structure is determined with a high-resistance voltmeter. This electrode has the advantages of low cost, good accuracy, and ruggedness.
12. Which of the following is/are the typical sacrificial anodes?
a) Magnesium only
b) Zinc and magnesium
c) Aluminum-tin only
d) Magnesium, Zinc, and aluminum-tin
View Answer
Explanation: Sacrificial anodes are metals that have a very high negative potential, which provides high current output. Magnesium, zinc, and aluminum-tin are typical examples of sacrificial anodes.
13. Which of the following is an impressed current anode that is efficient in a marine environment?
a) Platinized titanium
b) Silicon-iron
c) Scrap steel
d) Graphite
View Answer
Explanation: Platinized titanium is an inert anode that is both efficient and expensive in nature. It is finding increased applications in marine environments.
14. Which of the following statements is/are correct about stray current?
a) It usually encountered in cathodic-protection systems
b) It refers to extraneous direct current in the earth
c) Acceleration corrosion will occur at the point where the current enters the soil
d) Stray current refers to extraneous direct current in the earth that usually encountered in cathodic protection systems and accelerated corrosion will occur at the point where current enters the soil
View Answer
Explanation: Stray current refers to extraneous direct current in the earth that usually encountered in cathodic protection systems and accelerated corrosion will occur at the point where current enters the soil.
15. Which of the following is/are the preventions of stray current effect in an underground channel?
a) Use of bus conductor
b) Rearrangement of anodes
c) Use of bus conductor and rearrangement of anodes
d) Neither use of bus conductor nor rearrangements of anodes
View Answer
Explanation: Stray current effect caused due to the densely buried underground pipelines in the vicinity of the cathodic protection system. It can be prevented by the use of bus conductors and rearrangements of anodes.
Sanfoundry Global Education & Learning Series – Corrosion Engineering.
To practice all areas of Corrosion Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Mechanical Engineering Internship
- Check Corrosion Engineering Books
- Check Mechanical Engineering Books
- Apply for Metallurgical Engineering Internship
- Practice Metallurgical Engineering MCQs
