This set of Protein Engineering Questions and Answers for Aptitude test focuses on “Applications – Engineering Therapeutic Hormone : Insulin”.
1. Which of the following is the main reason for type 2 diabetes?
a) Insulin overproduction
b) Insulin deficiency
c) Insulin destruction
d) Insulin resistance
View Answer
Explanation: Insulin resistance is the main reason for type 2 diabetes. Type 2 diabetes (also known as adult-onset diabetes) is a lifelong disease in which the body becomes resistant to insulin. Insulin overproduction, Insulin deficiency, or Insulin destruction are not the main reasons for type 2 diabetes.
2. Which of the following is not true for a healthy person?
a) The pancreas secretes insulin continuously
b) Transient increase in insulin secretion
c) Beta cells secrete insulin
d) Insulin is only secreted after a meal
View Answer
Explanation: Healthy humans secrete insulin continuously at a low basal level, with rapid but transient increases triggered by elevated blood glucose concentrations. Insulin is secreted by beta cells of islets of Langerhans in the pancreas. Hence, the statement “Insulin is only secreted after a meal” is not true for a healthy person.
3. Which of the following consists of a 21 amino acid A-chain linked to a 30 amino acid B chain via two interchain disulfide linkages?
a) Somatostatin
b) Glucagon
c) Hemoglobin
d) Insulin
View Answer
Explanation: Insulin consists of a 21 amino acid A-chain linked to a 30 amino acid B chain via two interchain disulfide linkages. Insulin is a hormone made by the pancreas that allows the body to use glucose from the blood. Somatostatin, glucagon, or hemoglobin do not consist of a 21 amino acid A-chain linked to a 30 amino acid B chain via two interchain disulfide linkages.
4. Which of the following protein has a structure as depicted in the figure below?
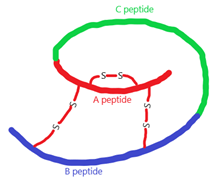
a) Glucagon
b) Hemoglobin
c) Insulin
d) Proinsulin
View Answer
Explanation: The structure depicted in the above figure is of proinsulin. Insulin is composed of two peptide chains referred to as the A chain and B chain, linked together by two disulfide bonds. An additional disulfide bond is formed within the A chain. Proinsulin is a single polypeptide chain precursor of insulin consisting of A chain, B chain, and C chain.
5. At physiological concentrations (10-10 M), insulin molecules exist in oligomeric form.
a) True
b) False
View Answer
Explanation: The above statement is false. At physiological concentrations (10-10 M), insulin molecules exist in monomeric form. However, when present at therapeutic dose concentrations (10-3 M) individual insulin molecules dimerize, with subsequent oligomerization of three dimers to form a hexamer, often co-ordinated with zinc ions.
6. A combination of fast-acting and slow-acting insulin must usually be administered to diabetics to mimic the natural state.
a) False
b) True
View Answer
Explanation: The above statement is true. A combination of fast-acting and slow-acting insulin must usually be administered to diabetics to mimic the natural state. Healthy humans secrete insulin continuously at a low basal level, with rapid but transient increases in insulin secretion triggered by elevated blood glucose concentrations.
7. Which of the following cells are destroyed in type 1 diabetes?
a) Immune cells
b) Gamma cell
c) Alpha cells
d) Beta cells
View Answer
Explanation: In type 1 diabetes, the immune cells of the body destroy insulin-producing cells (beta cells) in the pancreas. This condition is usually diagnosed in children and young people; therefore, it was called juvenile diabetes. Immune cells, alpha cells, and gamma cells are not destroyed in type 1 diabetes.
8. Which of the following amino acid cannot be inserted to minimize the monomer interactions?
a) Glutamate
b) Arginine
c) Lysine
d) Proline
View Answer
Explanation: Proline cannot be inserted to minimize monomer interactions. Glutamate, arginine, and lysine can be inserted to minimize monomer interactions. These amino acids are charged and hence cause repulsion when inserted in the peptide. Hence, these can be inserted to prevent monomer interactions.
9. Engineered slow-acting insulin is not fully soluble at which of the following pH?
a) pH 5.5
b) pH 5
c) pH 4
d) pH 7
View Answer
Explanation: Engineered slow-acting insulin is not fully soluble at pH 7, because this pH is close to its pI. Generally, proteins are least soluble at their pI values. Engineered slow-acting insulin is fully soluble at pH 5.5, pH 5, and pH 4.
10. Find the odd one out with respect to engineered fast-acting insulins.
a) Arginine
b) Aspartic acid
c) Histidine
d) Valine
View Answer
Explanation: Arginine, aspartic acid, and histidine are charged amino acids. These amino acids when inserted in the peptide chain of insulin significantly reduce the monomeric interactions. But valine is a neutral and nonpolar amino acid and hence it cannot reduce the monomeric interactions when inserted in the peptide chain of insulin. Therefore, valine is the odd one out.
11. Which of the following is the trade name of engineered slow-acting insulin?
a) Novorapid
b) Novolog
c) Novolin
d) Lantus
View Answer
Explanation: Lantus is the trade name of engineered slow-acting insulin. Novorapid and Novolog are trade names of engineered fast-acting insulin. Engineered slow-acting insulin mimic the ongoing basal insulin secretion characteristic of the normal state.
12. The introduction of amino acid substitutions that will discourage the monomer interaction is to be done inside the insulin receptor binding site.
a) True
b) False
View Answer
Explanation: The above statement is false. The introduction of amino acid substitutions that will discourage the monomer interaction is to be done outside the insulin receptor binding site. Therefore, this engineering of insulin will not alter the product’s biological activity/potency.
13. In Engineered slow-acting insulin (Lantus) the asparagine residue 21 of the A chain is replaced by glycine, and this chain is elongated at its C-terminal end by two arginine residues.
a) True
b) False
View Answer
Explanation: The above statement is false. In engineered slow-acting insulin (Lantus) the asparagine residue 21 of the A chain is replaced by glycine, and the B chain is elongated at its C-terminal end by two arginine residues. As a consequence, the molecule’s isoelectric point (pI) is increased from 5.4 to more neutral values.
14. Which of the following amino acid when inserted in the insulin chain significantly reduces the monomeric interactions?
a) Methionine
b) Valine
c) Glycine
d) Glutamic acid
View Answer
Explanation: Glutamic acid when inserted in the insulin chain significantly reduces the monomeric interactions, because of the negative charge on it. Valine, methionine, or glycine do not significantly reduce the monomeric interactions when inserted in the insulin chain.
15. Which of the following ion co-ordinates the oligomerization of three insulin dimers to form a hexamer?
a) Sodium ions
b) Calcium ions
c) Copper ions
d) Zinc ions
View Answer
Explanation: Zinc ions co-ordinate the oligomerization of three insulin dimers to form a hexamer. Sodium ions, calcium ions, or copper ions do not co-ordinate the oligomerization of three insulin dimers to form a hexamer. When present at therapeutic dose concentrations individual insulin molecules dimerize, with subsequent oligomerization of three dimers to form a hexamer, often co-ordinated with zinc ions.
Sanfoundry Global Education & Learning Series – Protein Engineering.
To practice all areas of Protein Engineering for Aptitude test, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Protein Engineering Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Check Biotechnology Books
