This set of Virology Multiple Choice Questions & Answers (MCQs) focuses on “Methods of Counting Viruses”.
1. Which of the following is an indirect method of counting animal viruses?
a) Counting using electron microscope
b) Counting using epifluorescence microscope
c) Counting using light microscope
d) Hemagglutination assay
View Answer
Explanation: Hemagglutination assay is an indirect method of counting animal viruses. Animal virus particles can bind to the surface of red blood cells. If the ratio of virus particles to cells is large enough, virions will join the red blood cells together causing agglutination. In this assay, the highest dilution of the virus preparation that can cause hemagglutination is determined.
2. Which lethal dose of the virus dilution is shown in the graph?
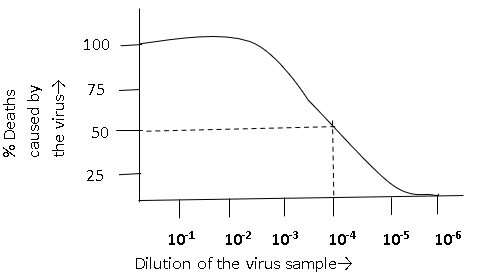
a) 10-4
b) 10-6
c) 10-5
d) 10-3
View Answer
Explanation: The dilution of virus preparation that contains a concentration of virions large enough to destroy 50% of the host cells or organisms is known as the lethal dose (LD50) of the virus. In the above graph, 50% of deaths occurred at 10-4 dilution of the virus sample. Therefore, the lethal dose of the virus is 10-4 dilution.
3. What is the infectious dose of a virus?
a) Dose that kills 50% of the host organisms
b) Dose that kills 25% of the host organisms
c) Dose that infects 50% of the host organisms
d) Dose that infects 25% of the host organisms
View Answer
Explanation: The infectious dose (ID50) is the dilution that contains a concentration (dose) of virus particles that causes 50% of the host organisms to become infected. When biological effects of the virus cannot be quantified by plaque and hemagglutination assays, the quantity of infectious virus particles required to cause a disease is determined by this method.
4. The principle of which of the following assays is used to determine the concentration of virions (infectious virus particles) in plants?
a) Plaque assay
b) Hemagglutination assay
c) LDH assay
d) MTT assay
View Answer
Explanation: The approach of plaque assay is slightly modified and used for determination of virus concentration in plants. In this process, plant leaves are inoculated with a diluted preparation of virus particles. The number of necrotic lesions formed on the leaves after infection is used to calculate the concentration of infectious virus particles.
5. A student wants to dilute a virus sample. He adds 1 ml of the given sample to test-tube A containing 9ml of water. Then, he takes 1ml of liquid from test-tube A and adds it to test-tube B containing 9ml of water. What is the total dilution factor of the sample in test-tube B?
a) 10-1
b) 10-2
c) 10-3
d) 10-4
View Answer
Explanation: We know, Dilution factor = Volume of sample/ (Volume of sample + Volume of water)
Dilution factor of test-tube A = 1ml/ (1ml + 9ml)
= 1ml/10ml
= 10-1
Similarly, dilution factor of test-tube B = 1ml/ (1ml + 9ml)
= 1ml/10ml
= 10-1
Therefore, total dilution factor of the sample
= Dilution factor of test-tube A*Dilution factor of test-tube B
= 10-1*10-1
= 10-2
6. What is the lethal dose of the virus whose lethality is shown in the below table?
| Dilution of the virus | Total number of hosts | Number of hosts alive | Number of hosts dead |
| 10-6 | 20 | 5 | 15 |
| 10-7 | 20 | 8 | 12 |
| 10-8 | 20 | 10 | 10 |
| 10-9 | 20 | 15 | 5 |
a) 10-9
b) 10-8
c) 10-7
d) 10-6
View Answer
Explanation: The dilution of virus that contains a concentration of infectious virus particles that can kill 50% of the host organisms is called the lethal dose of the virus.
At dilution 10-6, percentage of dead hosts = (No. of hosts dead/Total no. of hosts) *100
= (15/20) *100
= 75
At dilution 10-7, percentage of dead hosts = (12/20) *100
= 60
At dilution 10-8, percentage of dead hosts = (10/20) *100
= 50
At dilution 10-9, percentage of dead hosts = (5/20) *100
= 25
Therefore, dilution 10-8 is the lethal dose of the virus because 50% of the hosts died in that dilution.
7. A sample of bacterial viruses was used to inoculate bacterial cells in a culture. After incubation, no death of cells was observed. Which of the following can be the cause of this problem?
a) The sample was too diluted
b) The sample was too concentrated
c) The sample had intermediate dilution
d) Viruses cannot kill bacterial cells
View Answer
Explanation: When more concentrated virus sample is used to inoculate cells in a culture, all cells of the culture lyse and die. When a virus sample of intermediate dilution is used, only the cells that received virus particles die. When a very diluted virus sample is used, none of the cells are killed because the cells do not receive any infectious virus particles. Therefore, the sample used in the experiment was too diluted.
8. Which of the following viruses can be quantified using focus-forming assay?
a) Viruses that kill the cells
b) Viruses that cannot infect the cells
c) Viruses that are not present in the culture
d) Viruses that change morphology of the infected cells
View Answer
Explanation: Focus-forming assay is used to quantify the viruses that can transform the morphology of the infected cells. A single infectious virus particle can lead to the formation of a discrete colony of cells. The number of such colonies present in the culture is counted to measure the quantity of infectious virus particles present.
9. Which of the following stains can be used to visualize host cells during immunofluorescence assay?
a) Methylene blue
b) Safranin
c) DAPI
d) Eosin
View Answer
Explanation: DAPI is a stain that binds to the A-T rich region of the DNA and gives blue fluorescence. DAPI is used in immunofluorescence assay to stain the nuclei of the host cells. Immunofluorescence assay uses tagged antibodies (gives green fluorescence) for detection of viral proteins in the infected cells. So, after the assay all cells have blue-stained nuclei which can be visualized by fluorescence microscope and the infected cells show green color.
10. Which of the following is detected in virus neutralization assay?
a) Antigen
b) Antibody
c) Nucleic acid
d) Lipid
View Answer
Explanation: Virus neutralization assay is used to detect the antibody that can block virus replication. It is a special type of immunological assay because it cannot detect all antigen-antibody reactions. Only the antibodies, which are capable of neutralizing virus infections, can be detected by this method.
11. Virus preparations of different dilutions, which were prepared from the same virus sample, were used to inoculate the bacterial cultures A, B, C and D. Which culture plate was inoculated with the most dilute virus preparation?
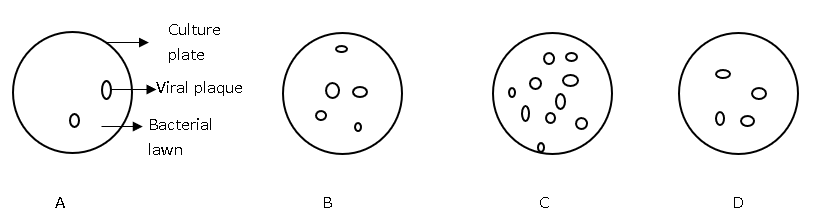
a) A
b) B
c) C
d) D
View Answer
Explanation: We know, the number of clear spots or plaques formed on the bacterial lawn, after virus infection, is inversely proportional to the dilution of virus preparation added. In the diagram, culture plate A has the least number of plaques. Therefore, it was inoculated with the most dilute virus preparation.
12. What will happen to the number of plaque-forming units (PFU) if the number of virions in a virus culture is doubled?
a) The number of PFU will be equal to the total number of virions
b) The number of PFU will be half of the initial value
c) The number of PFU will be twice of the initial value
d) The number of PFU will be zero
View Answer
Explanation: A complete virus particle is called a virion. The number of PFU is proportional to the number of virions. Therefore, a preparation with twice as many virions will have twice the PFUs. The number of PFU will not be equal to the number of virions because not all virions may be infective.
13. Which of the following methods can be used to quantify the nucleic acid of virus?
a) Electron microscopy
b) Epifluorescence microscopy
c) Quantitative-polymerase chain reaction(qPCR)
d) Plaque assay
View Answer
Explanation: The qPCR amplifies specific nucleic acids of the virus in a mixture of nucleic acids and quantifies the nucleic acid concentration. Electron microscopy and epifluorescence microscopy are direct methods of counting animal viruses. Plaque assay is an indirect method of counting virion numbers.
14. Which of the following methods is used for purification of plant viruses?
a) Ultracentrifugation of plant sap
b) Enzyme treatment
c) Inoculation of plant
d) Microscopic study
View Answer
Explanation: Ultracentrifugation causes separation of the virus particles from the host cell components and concentrates the virus. The purified virus is obtained as a colorless pellet in a test tube after ultracentrifugation. The purified virus thus obtained can be used for qPCR (Quantitative polymerase chain reaction) to quantify its nucleic acid content. It can also be used to determine the number of virus particles present in the preparation using different direct and indirect counting methods.
15. Noncytopathic viruses present in a culture cannot be detected.
a) True
b) False
View Answer
Explanation: The viruses that do not kill the infected cells are called noncytopathic viruses. The cells infected by noncytopathic viruses can be recognized by the presence of virus protein or nucleic acid. Specific detection reagents are used to detect the nucleic acids and proteins produced by the viruses.
Sanfoundry Global Education & Learning Series – Virology.
To practice all areas of Virology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Virology Books
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
