This set of Nanotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Chemistry of Fullerene”.
1. All fullerenes obey the isolated pentagon rule.
a) True
b) False
View Answer
Explanation: The isolated pentagon rule states that fullerenes having isolated pentagon are kinetically more stable than their fused pentagon counterparts. In general, fullerenes with less than 60 carbon atoms do not obey the IPR.
2. Choose the incorrect reaction.
a)
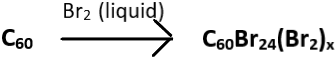
b)
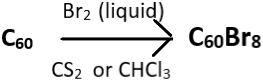
c)
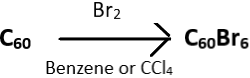
d)
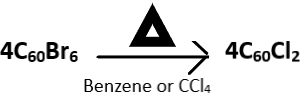
View Answer
Explanation: The brominated fullerene, C60Br6 dissociates into C60Br8 and C60 upon heating in the presence of carbon tetrachloride (CCl4) or benzene. C60Br8 has higher stability than C60Br6 and this attributed to the lack of eclipsing interactions of C60Br8 present in C60Br6. The correct reaction is given below as:
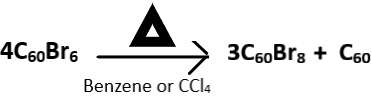
3. Which of the following is not one of the requirements for producing fulleride salts by electro crystallization?
a) Insolubility of salt in solvent
b) Counter cation
c) Extremely high solubility of salts in solvent
d) Proper solvent
View Answer
Explanation: C60 anions can be electrochemically produced in a defined oxidation state by applying a proper potential. This is used for the production of fulleride salts by electro crystallization. The process demands for the insolubility of the salt in the solvent. It can be achieved by using a proper solvent, oxidation state of C60 and counter cations which usually come from the supporting electrolyte.
4. Why doesn’t C60 react with Hg2I2/Hg in benzonitrile for concentration of (THA)I at 1mM?
a) Hg2I2/Hg is not sufficiently negative
b) C60 is inert towards Hg2I2/Hg
c) (THA)I is incompatible with benzonitrile
d) Very high concentration of (THA)I
View Answer
Explanation: The reaction of C60 and Hg2X2/Hg couple strongly depend on the solvent. When C60 is made to react with a drop of mercury in 1mM (THA)I in presence of benzonitrile, no reaction is observed. This is because the potential of the (Hg2I2/Hg) couple under the given conditions is not sufficiently negative to permit the reduction of C60. An increase in the (THA)I concentration to 0.1M, however, leads to the reaction between the reactants.
5. Which of the following is an example of Bingel reaction?
a) Reaction of 1,3 – butadiene with ethene
b) Reaction of pyrrolidinofullerene with maleic acid in presence of Wilkinson’s catalyst
c) Reaction of alkenes with a 1,3 – dipolar compound
d) Reaction of C60 with a tether connected bismalonate in presence of I2 and DBU
View Answer
Explanation: When C60 is made to react with a tether – connected bismalonate in presence of iodine and DBU, corresponding 2-iodo-malonate is formed in situ. This, reacts with C60. The reaction is an example of Bingel reaction. The functionalization provides improved yields and regioselectivity.
6. Choose the correct order for the preparation of alkaline earth metal fullerides.
a) Mixing of C60 and metal ➔ Heat treatment of sample ➔ Compression of powder into pellets ➔ Placing powder in tantalum cell
b) Mixing of C60 and metal ➔ Placing powder in tantalum cell ➔ Compression of powder into pellets ➔ Heat treatment of sample
c) Heat treatment of sample ➔ Compression of powder into pellets- ➔ Mixing of C60 and metal – ➔ Placing powder in tantalum cell
d) Heat treatment of sample ➔ Mixing of C60 and metal ➔ Placing powder in tantalum cell ➔ Compression of powder into pellets
View Answer
Explanation: Alkaline earth metals are mixed with C60 in an inert atmosphere. The mixture powder is placed in a high purity tantalum cell, which allows compression into pellets. These pellets are loaded into quartz tubes, being subsequently sealed under vacuum. Finally, the samples are heated between 550°C – 800°C for periods ranging from hours to weeks. This leads to the corresponding fullerides.
C60+ 5Ca ➔ Ca5C60
7. How can C60Cl6, a single product, be synthesized using C60?
a) By reacting C60 with an excess of iodine monochloride in benzene
b) By reacting C60 with chlorine at -35°C
c) By reacting C60 with aluminium trichloride in benzene
d) By reacting C60 with XeF2 in dichloromethane solutions
View Answer
Explanation: C60Cl6, a chlorofullerene can be produced singly by the reaction of C60 with an excess of iodine monochloride in benzene or toluene at room temperature.
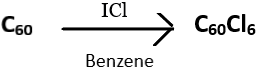
The formation of C60Cl6 using toluene, as a solvent, is slower than with benzene. This indicates that radicals are involved in the reaction and they are scavenged by the toluene.
8. What is the mistake in the given reaction?
nMBH4 + C60 ➔ MnC60 + nH2 + n/2(B2H6)
a) Stoichiometry of hydrogen is unbalanced
b) Stoichiometry of metals is unbalanced
c) Stoichiometry of carbon is unbalanced
d) Stoichiometry of boron is unbalanced
View Answer
Explanation: Alkali metal fullerides, MnC60, can be produced by the reaction of solid C60 with solid MH or nMBH4. Here, M is Na or K.
nMBH4 + C60 ➔ MnC60 + (n/2)H2 + (n/2)(B2H6)
The advantage of this reaction is the easier handling of small amounts of MBH4 in comparison to alkali metals.
9. Why is complete hydrogenation of fullerene hypothetical?
a) Excessive strain
b) Highly stable
c) Formation of fullerene cage
d) Low temperature conditions
View Answer
Explanation: Fullerenes tend to undergo hydrogenation to form products such as C60H18, C60H36 etc. But complete hydrogenation of fullerene to form C60H60 is only hypothetical. This is due to the large strain in the structure. Moreover, highly hydrogenated fullerenes tend to be unstable owing to cage fragmentation. This happens when fullerenes are made to react with hydrogen directly at high temperatures for a longer time.
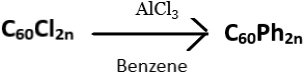
10. What does the following reaction yield?
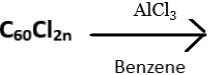
a) C60
b) C60Al
c) C60Ph2n
d) C60PhAl
View Answer
Explanation: Polyfullerenes, C60Cl2n, can undergo Friedel – Crafts type reactions. C60Cl2n when treated with a catalytic amount of aluminium chloride in benzene solutions at room temperature for about two hours, results in the formation of polyphenylated fullerenes. The product obtained has at least 22 phenyl groups attached to the fullerene core.
11. What is IPR(isolated pentagon rule)?
a) Pentagons in fullerenes should possess double bonds
b) Pentagons in fullerene when remain isolated are kinetically less stable
c) Pentagons in fullerene do not share an edge
d) Pentagons in fullerene shows excellent delocalization of electrons
View Answer
Explanation: The IPR was first proposed by Kroto in 1987. It stated that the most stable fullerenes are those in which no two pentagons share an edge which indicates that each pentagon is completely surrounded by hexagons. This could also mean that the fullerenes with isolated pentagons are kinetically more stable than their fused pentagon counterparts.
12. How can self – assembled monolayers (SAMs) of covalently bound C60 be synthesized?
a) C60 treated with (MeO)3 Si modified indium-tin-oxide (ITO) surfaces
b) C60 treated with Me3Si(CH2)3 modified gold surfaces
c) C60 treated with MeOSi3CH2 modified gold surfaces
d) C60 treated with (MeO)3Si(CH2)3 modified indium-tin-oxide (ITO) surfaces
View Answer
Explanation: The formation of self-assembled monolayers (SAMs) take place when C60 is treated with (MeO)3Si(CH2)3 modified indium-tin-oxide (ITO) surfaces. It can also be synthesized by treating C60 with cysteamine modified gold surfaces.
13. Choose the incorrect statement from the following options.
a) Fluorination of fullerenes is an endothermic process
b) Polyfullerenes are insoluble in water
c) Color of polyfullerenes vary between brown and white
d) Fluorinated fullerenes are susceptible to nucleophilic substitutions
View Answer
Explanation: Fullerenes undergo halogenation reactions. Fluorination of fullerenes is an exothermic process. Polyfullerenes formed as a product are very soluble in toluene, THF etc., but remain insoluble in water. Moreover, the fluorinated fullerenes are susceptible to nucleophilic substitutions. The colors of the polyfullerenes, C60F2n, vary between brown and white in accordance with the procedure and the degree of fluorination.
14. What are fullerenes having metal atoms enclosed inside their cages called?
a) Endohedral metallofullerenes
b) Heterofullerenes
c) Exohedral metallofullerenes
d) Fullerene polymers
View Answer
Explanation: Endohedral fullerene complexes are the ones in which one or more metal atoms are present inside the fullerene cages. These fullerene complexes can be produced by pulsed laser vaporization of metal oxides / graphite composite rod in argon gas at 1200°C. Arc vaporization of graphite, saturated with various metal oxides can also prove to be an effective method for synthesis of endohedral metallofullerenes.
15. Which of the following reactions do not result in alkali metal fullerides?
a) Reaction between C60 and Ba
b) Reaction between C60 and MH
c) Reaction between C60 and Rb
d) Reaction between C60 and MBH4
View Answer
Explanation: Alkali metal fullerides are obtained by the reaction of C60 with alkali metals such as sodium, potassium, rubidium and cesium in liquid ammonia. They can also be formed when C60 reacts with metal hydrides or metal borohydrides. But when C60 reacts with barium (Ba), alkaline earth metal fullerides are formed.
Sanfoundry Global Education & Learning Series – Nanotechnology.
To practice all areas of Nanotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
