This set of Nanotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Chemistry of Fullerene – Set 2”.
1. What are the reaction conditions for the catalytic hydrogenation of C60?
a) Temp = 800°C – 900°C; Pressure = 1bar; Radical promoter = iodomethane
b) Temp = 600°C – 750°C, Pressure = 0.1MPa; Radical promoter = bromoethane
c) Temp = 400°C – 450°C, Pressure = 70bar; Radical promoter = iodoethane
d) Temp = 50°C – 100°C; Pressure = 6MPa; Radical promoter = bromomethane
View Answer
Explanation: Fullerenes such as C60 and C70 are placed in a glass vessel inside an autoclave. These contain an excess of iodoethane and remain pressurized with hydrogen to 6.9 MPa or 69 bar. The process also requires a temperature range of about 400 – 450°C for one hour.
2. Why isn’t C60 super aromatic?
a) Occurrence of excellent electron delocalization
b) Electron deficient alkene
c) Pentagonal rings have double bonds within
d) It follows Hirsch’s rule for aromaticity
View Answer
Explanation: Fullerenes, such as C60, have a tendency to avoid double bonds within pentagonal rings which limits the delocalization of electrons to a great extent. Because of the poor delocalization, C60 cannot show super aromaticity. It is spherically π – antiaromatic and is a highly strained species.
3. Complete the given reaction.
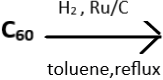
a) CnH2n+1
b) C60Hn
c) CnH2n-1
d) CnH2n
View Answer
Explanation: Fullerenes undergo catalytic hydrogenations to give hydrofullerenes. C60 upon reacting with hydrogen, in the presence of activated carbon with ruthenium as a catalyst and in refluxing toluene, forms C60H2n. This results in comparatively higher degrees of hydrogenation (upto C60H50). The given reaction is
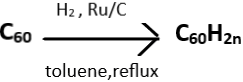
4. Fullerenes behave as electrophiles and hence can only undergo nucleophilic additions.
a) True
b) False
View Answer
Explanation: Fullerenes tend to react as electrophiles. They undergo both electrophilic and nucleophilic additions. For instance, C60 when electro-reduced in solutions of benzonitrile and TBAClO4, and further treated with excess MeI resulted in the formation of 1, 2 – C60 (CH3)2 and 1, 4 – C60 (CH3)2. This is an electrophilic addition. Nucleophilic additions of fullerenes include Bingel reaction.
5. What product is obtained when C60 is reacted with nitric acid and sulphuric acid followed by an aqueous base?
a) Hydrogenated fullerenes
b) Hydroxylated fullerenes
c) Oxygenated fullerenes
d) Chlorinated fullerenes
View Answer
Explanation: When C60 is made to react with sulphuric acid and nitric acid followed by the hydrolysis of the intermediates with aqueous base, fullerols or fullerenols or Hydroxylated fullerenes are formed.
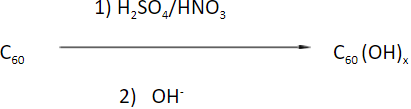
6. How does K3C60 behave at temperatures in the order of 10 – 40 K?
a) Conductor
b) Semiconductor
c) Insulator
d) Superconductor
View Answer
Explanation: Alkali metal doped C60 exhibits superconductivity. K3C60 was the first alkali metal doped fullerene. This compound behaves as a superconductor in the temperature range of 10 – 40 K. Precisely, the transition to a superconducting state for this compound occurs at critical temperature, Tc = 19.3K.
7. Why are fullerenes electronegative?
a) Exhibit super aromaticity
b) Empty low lying π* orbitals exhibit high s – character
c) Severe extension of the p-lobes into the sphere of fullerene
d) Presence of delocalized electrons that conduct electricity
View Answer
Explanation: Fullerenes are electronegative in nature. This is because the empty low lying π* orbitals have high s-character. The other reason behind their electronegativity is that the p-lobes in sp2 hybridization extend further outside the surfaces than they do into the interior of the sphere.
8. This is an example of what kind of reaction?
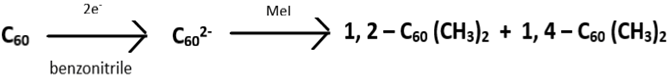
a) Nucleophilic addition
b) Cycloaddition
c) Hydroxylation
d) Electrophilic addition
View Answer
Explanation: Fulleride anions undergo electrophilic additions. For instance, C60 in presence of benzonitrile solutions and tetra-tert-butylammonium perchlorate (TBAClO4) when electro-reduced resulted in C602-. This again on treatment with an excess MeI, after turning off the potential, results in the formation of C60 (CH3)2. The dimethyldihydrofullerenes-60 isolated from the reaction mixture have isomers of (1, 2-) and (1, 4-) formed in the ratio of 1.4:1.
9. Heating C60/C70 mixtures in toluene with excess KOH results in the formation of which of the following compounds?
a) Alkali metal fullerides
b) Fullerols
c) Hydrofullerenes
d) Charged amino radical
View Answer
Explanation: When C60/C70 mixtures are heated in toluene in the presence of excess KOH, precipitation of hydroxylated fullerenes (fullerols) takes place. The product formed is soluble in THF but decompose in the presence of air. Here, addition of hydroxide to C70 is much faster than to C60.
10. What is the given reaction known as?
![]()
a) Bechamp reduction
b) Clemmensen reduction
c) Birch-Hϋckel reduction
d) Bouveault-Blanc reduction
View Answer
Explanation: Birch-Hϋckel reduction is the most widely used method for producing hydrofullerenes. The reduction process is carried out with lithium in liquid ammonia in the presence of tert-Butyl alcohol. There is a distinct change in colour observed during the reaction from purple C60 to a light cream to off white substance.
Sanfoundry Global Education & Learning Series – Nanotechnology.
To practice all areas of Nanotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
