This set of Nanotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Structural Characterization of Graphene”.
1. Trilayer graphene exhibits higher mobility than monolayer and bilayer graphene.
a) True
b) False
View Answer
Explanation: With the increase in the layers of graphene, its effective mass also increases. As a result the trilayer graphene having greater layer thickness exhibits lower mobility than those of monolayer and bilayer.
2. How many σ and π bonds does each atom of carbon have in graphene lattice?
a) 3σ bonds and 1π bond
b) 1σ bond and 3π bonds
c) 2σ bonds and 2π bonds
d) 4σ bonds and 0π bond
View Answer
Explanation: Each atom of carbon in graphene lattice is bonded to three other carbon atoms through covalent bonds, forming sigma bonds with them. The remaining electrons of each atom present in an unhybridised p-orbital form the π bonds.
3. What is the bond length of the σ-bond formed between two carbon atoms in the graphene lattice?
a) 1.72 Å
b) 1.99 Å
c) 1.42 Å
d) 1.10 Å
View Answer
Explanation: Each carbon atom in graphene lattice is bonded by covalent σ-bonds to three other neighboring carbon atoms. These σ-bonds have a bond length of 1.42 Å or 1.42 × 10-10m each.
4. What is the hybridization exhibited by carbon atoms in graphene lattice?
a) sp3
b) sp2
c) sp3d2
d) sp3d
View Answer
Explanation: Carbon atoms in graphene lattice exhibit sp2 hybridization. The high stability of graphene is due to the tightly packed carbon atoms and sp2 orbital hybridisation. This is a combination of s, px and py orbitals.
5. Why is electronic structure of graphene nanoribbons (GNRs), having ultrathin width of 10nm, sensitive to the edge orientations?
a) Greater surface interactions with the surroundings
b) Reduced surface free energy
c) Decreased surface interactions with the surroundings
d) Presence of different edge orientations
View Answer
Explanation: The surface free energy of a material increases with its decreasing size and thus its properties start depending upon the surface interactions. This size effect is more prominent incase of nanomaterials having size in the range of 1-100nm. This is due to the fact that most of their atoms are exposed to the surface and interact with the surroundings. Hence, electronic structures of ultra-thin GNRs are sensitive to their edge orientations.
6. Graphene is an amorphous allotrope of carbon.
a) True
b) False
View Answer
Explanation: Allotropy is a property shown by certain chemical elements in which they exist in two or more different physical forms. Carbon has both amorphous and crystalline forms of allotrope. Graphene is a crystalline two dimensional carbon allotrope. Other crystalline allotropes of carbon include diamond, buckyballs, and carbon nanotubes.
7. Which of the following geometrics correspond to the hybridisation of Graphene?
a)
![]()
b)
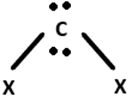
c)
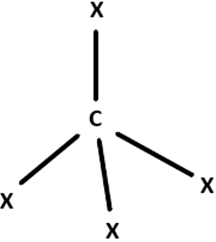
d)
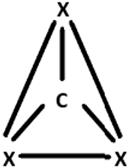
View Answer
Explanation: Graphene exhibits sp2 hybridisation that is a combination of s, px and py orbitals that forms the 3 σ-bonds with 3 other neighboring carbon atoms. The pz orbital remains oriented out of the plan, which forms the π-bond.
8. What does not account for graphene’s stability?
a) Tightly packed atom
b) σ-bond
c) Amorphous nature
d) sp2 hybridisation
View Answer
Explanation: Graphene has great stability owing to its structure. The sp2 orbital hybridisation that is the combination of s, px and py orbitals deliver immense strength to it. These orbitals constitute the σ-bonds. Graphene’s high stability and tensile strength is also due to the tightly packed carbon atoms in its lattice.
9. What forces act between graphene multilayers?
a) Gravitational forces
b) Spring forces
c) Vander Waals forces
d) Frictional force
View Answer
Explanation: Graphene’s structure has π-bonds in it that provide weak Vander Waal interactions between adjacent layers. These Van der Waal forces allow the layers to glide over each other.
10. How can graphene self-repair the holes in its sheets?
a) When it is exposed to carbon containing molecules
b) When it is exposed to nitrogen containing molecules
c) When it is exposed to sulphur containing molecules
d) When it is exposed to phosphorus containing molecules
View Answer
Explanation: Graphene has the ability to self-repair holes present in its sheet, when it is exposed to carbon containing molecules. The carbon atoms are bombarded at the graphene sheets that are torn and as a result these atoms align perfectly into hexagons, thereby completely filling the holes.
11. Which of the following is incorrect regarding the edge orientations of GNRs?
a) In tight-binding theory, zig-zag GNRs are always metallic
b) GNRs having greater zig-zag edges than armchair show smaller band gap
c) In tight-binding theory, arm-chair GNRs are either metallic or semiconducting
d) Energy band gaps of semiconducting armchair GNRs decrease with the decrease in their width
View Answer
Explanation: Armchair GNRs manifest metallic or semiconducting behavior that depends on their width. The energy band gaps, for instance, of the semiconducting armchair GNRs increase as their width decreases.
12. Choose one of the following properties in Graphene that does not suit for its use in composite material.
a) High surface area to volume ratio
b) Presence of disorders in lattice
c) High tensile strength
d) Presence of free electrons
View Answer
Explanation: Graphene has high tensile strength and high surface area to volume ratio. It also has a number of free electrons in its lattice structure that have the capability to bond with other atoms. These features of graphene make it useful for its application in composite materials.
13. What is the ground state electronic configuration of graphene?
a) 1s2 2s2
b) 1s2 2s2 2px2 2py1 2pz1
c) 1s2 2s2 2px1 2py1 2pz0
d) 1s2 2s2 2px2 2py2 2pz2
View Answer
Explanation: Graphene is comprised of carbon atoms. The ground state electronic configuration of carbon atom is (1s2 2s2 2px1 2py1 2pz0) which indicates the presence of 4 electrons in s-orbital and 2 electrons in p-orbitals.
14. Which of the following is not influenced by the different stacking arrangements of the honey comb network of planar structures in graphene films?
a) Spin-orbit coupling
b) Surface free energy
c) Band structure
d) Interlayer screening
View Answer
Explanation: There are countless stacking sequences in case of graphene sheets. These different stacking arrangements of the honey comb network, forming phase structures, strongly influence the interlayer screening, band structure and spin-orbit counting of the resulting graphene films.
15. Which of the following properties of graphene might not be useful in making touch screens?
a) Electrical conductivity
b) Transparency
c) Flexibility
d) Brittleness
View Answer
Explanation: Graphene has high potential for its use in electronic devices that are ultra thin. The electrical conductivity, transparency, flexibility and strength of graphene make it highly useful for future touch screens and ultra-thin electronic devices.
Sanfoundry Global Education & Learning Series – Nanotechnology.
To practice all areas of Nanotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
