This set of Nanotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Nanoparticles”.
1. Select the correctly matched pair from the following options.
a) Sol=Whipped cream; Emulsion=Smoke
b) Foam=Fog; Gel=Agar
c) Aerosol=Lava; Sol=Ice cloud
d) Emulsion=Latex; Foam=Soda suds
View Answer
Explanation: Colloids are classified on the basis of their dispersed phase and the medium of the dispersion. Emulsion is a colloid between two or more liquids with both the dispersion medium and phase being liquid. Example: – Latex, oil in water. Foam is a colloid formed when gaseous particles are dispersed in solid or liquid. Example: – Soda suds, whipped cream.
2. Choose the correct statement from the following.
a) Tyndall effect is observed due to dispersion of light
b) Tyndall effect is observed due to polarization of light
c) Tyndall effect is observed due to scattering of light
d) Tyndall effect is observed due to diffraction of light
View Answer
Explanation: Tyndall effect is observed when colloidal particles scatter light upon passing a beam of light through the colloidal solution. The scattering of light illuminates the path of the beam that is it makes the path visible in the colloidal dispersion.
3. Blood is a true solution.
a) True
b) False
View Answer
Explanation: Blood is a colloidal solution of an albuminoid. It is a bio-colloid. Blood has corpuscles dispersed in serum and these corpuscles have a size ranging between 1nm to 100nm.
4. There are three substances A, B and C with coagulation values as 5, 2.1, and 0.06 for a metal sol respectively. Find out the ratio of their coagulating powers.
a) 12.92 : 5 : 0.095
b) 0.2 : 0.476 : 16.666
c) 3.33 : 0.623 : 1
d) 0.0267 : 0.08 : 3
View Answer
Explanation: The flocculating power is inversely proportional to the coagulation value.
Now according to the question, A, B, C have coagulation values as 5, 2.1 and 0.06 for a metal sol respectively.
We know, Flocculation power ∝ (1/coagulation value)
A ∝ (1/5) = 0.2;
B ∝ (1/2.1) = 0.476;
C ∝ (1/0.06) = 16.666
Hence, A : B : C = 0.2 : 0.476 : 16.666.
5. Find out a method from the given options that is not used for the preparation of colloids.
a) Double decomposition
b) Vulcanization
c) Peptization
d) Hydrolysis
View Answer
Explanation: Vulcanization involves a series of processes for hardening rubbers. It is originally referred to the treatment of natural rubber with sulphur in order to increase its rigidity and durability and also alter its mechanical and electrical properties.
6. How can we obtain colloidal dispersion in organic liquids?
a) Destabilize a metallic core using halogens
b) Stabilize a metallic core using lipobhobic surfactants
c) Stabilize a metallic core using NR4Cl
d) Destabilize a metallic core using lipophilic surfactants
View Answer
Explanation: Colloidal dispersion in organic liquids can be obtained by stabilizing a metallic core using a lipophilic surfactant NR4X. Here, X is a halogen such as chlorine (Cl) or bromine (Br) while R represents the alkyl group CnH2n+1. This is a hydrocarbon radical obtained by removing a hydrogen atom from the terminal carbon atom in a straight – chain alkane, having n in the range of 6-20.
7. Glucose is frequently used as diluents in the formation of which of the following?
a) Benzosols
b) Sulpher sols
c) Soap sols
d) Gold sols
View Answer
Explanation: During the preparation of sulphur sols by mechanical dispersion method glucose is added as a diluent. This is an inert diluent which prevents the colloidal particles in the sol to grow in size.
8. Which of the following reactions does not result in colloid formation?
a) 5HI + HIO3 ➔ 3I2 + 3H2O
b) AuCl3 + 3SnCl2 ➔ 2Au + 3SnCl4
c) H2S + Br2 ➔ S + 2HBr
d) FeCl3 + 3H2S ➔ Fe(OH)3+ 3HCl
View Answer
Explanation: The given reaction of,
5HI + HIO3 ➔ 3I2 + 3H2O
is a halogenation reaction. It cannot prepare a colloid. However, the other reactions are various condensation methods used for the preparation of hydrophobic colloidal sols.
9. What is the phenomenon of migration of colloidal particles under the influence of a spatially uniform electric field known as?
a) Electrophoresis
b) Electrodialysis
c) Electro-osmosis
d) Electroluminescence
View Answer
Explanation: Electrophoresis refers to the migration of colloidal particles under the influence of a spatially uniform electric field. The motion of positively charged particles is known as cataphoresis while the movement of negatively charged particles is called anaphoresis.
10. Which of the following is not a colloid?
a) Cytoplasm of living cells
b) Mixture of sand and water
c) Coloured glasses
d) Mayonnaise
View Answer
Explanation: Mixture of water and sand is an example of a suspension. The difference between a colloid and a suspension is the size of the particles and also that the particles will not settle to the bottom over a period of time and they will stay suspended or float.
11. Which of the following is the Lennard – Jones equation?
a) dG1 = (A/R12) – (B/R6)
b) dN1(t)/dt = -λ1N1(t)
c) fw(Sw) = λw/(λw+λn)
d) ∂n/∂t = – (1/ϵ0)∇.qs
View Answer
Explanation: The Lennad Jones equation is given as;
dG1 = (A/R12) – (B/R6)
where dG1 is the interaction energy between colloidal particles. A and B are constants while R is the distance between two particles. In this equation, first term denotes short range repulsive interaction (Born repulsive interaction). The second term, on the other hand, indicates long range attractive interaction.
12. Which method of colloid formation is represented by the given diagram?
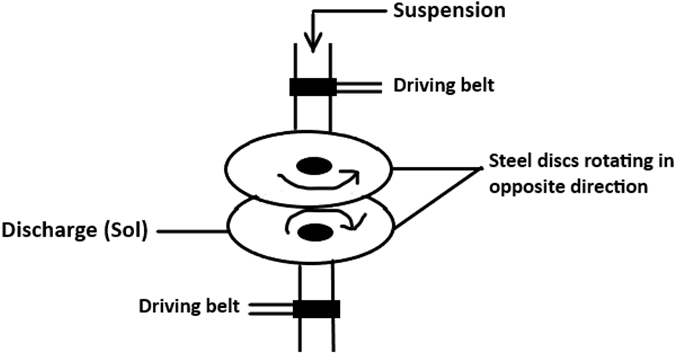
a) Mechanical dispersion method
b) Peptization
c) Bredig’s Arc method
d) Condensation method
View Answer
Explanation: The given schematic diagram represents the mechanical dispersion method of colloid formation. It shows a colloid mill comprising of two heavy steel discs that remain separated by a gap. This is adjusted with respect to the size of particle desired. The discs are rotatable at high speed in opposite direction.
13. Choose the correct statement from the following options.
a) Colloids do not flocculate
b) True solutions exhibit Tyndall scattering
c) Colloids fail to show Brownian motion
d) THF is used to stabilize metallic nanoparticles as colloids
View Answer
Explanation: Colloids show Brownian motion. They also exhibit Tyndall effect. They undergo flocculation when some electrolyte is added to them. It has been observed that ring compounds such as tetrahydrofuran (THF) and tetrahydrothiophene can be used to stabilize metallic nanoparticles as colloids.
14. Why does smoke emitted by motorcycles have a blue tinge?
a) Scattering of light
b) Polarization of light
c) Reverse osmosis
d) Electrodialysis
View Answer
Explanation: Smoke emitted by motorcycles generally has a blue tinge. This is due to the scattering of light by the particles provided by the machine when the engine oil burns. During the scattering, longer wavelengths are more transmitted than the shorter wavelengths that are more diffusely reflected by scattering.
15. Which of the following is not a factor affecting the electrostatic interactions between colloidal particles?
a) Mass of the particles
b) Surface potential
c) Mobility of the phases
d) Screening length of solvent
View Answer
Explanation: In general, electrostatic interactions between colloidal particles depend upon the mobility of the phases, surface potential and screening length of the solvent. It also depends upon the charges of both the dispersed phase and the dispersion medium.
More MCQs on Nanoparticles:
- Nanoparticles MCQ (Set 2)
- Nanoparticles MCQ (Set 3)
- Nanoparticles MCQ (Set 4)
- Nanoparticles MCQ (Set 5)
Sanfoundry Global Education & Learning Series – Nanotechnology.
To practice all areas of Nanotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
