This set of Nanotechnology Multiple Choice Questions & Answers (MCQs) focuses on “Chemical Characterization of Graphene”.
1. Why is the reactivity of edges in graphene sheets sensitive to carbon termination like zigzag and armchair?
a) High degree of curvatures in graphene sheets
b) Presence of hydrogen atoms in graphene sheets
c) Delicate competition of energy per atom
d) High height to radius ratio for corrugations
View Answer
Explanation: The chemical reactivity of graphene edges is crucial and sensitive to the graphene edge topologies, either zigzag or armchair. This is because of the delicate competition of energy per atom and their density.
2. How can the local reactivity be increased in Graphene?
a) Introduction of defects with dangling bonds
b) Introduction of Stone Wales (SW) defects
c) Removal of dopants
d) Removal of external mechanical strains
View Answer
Explanation: Reconstructed defects without dangling bonds, such as stone wales and divacancies have immensely low mobility. These defects have the possibility of increasing the local reactivity due to locally changed density of π-electrons.
3. Defect free graphene sheets are chemically inert.
a) True
b) False
View Answer
Explanation: Defects, if present in a graphene sheet, increases its chemical reactivity. Defect free sheets seem to be chemically inert, and these surfaces usually interact with other molecules by physical adsorption (π-π) interactions.
4. Which among the following options is incorrect regarding ways of increasing the chemical reactivity of graphene?
a) Reaction with halogen atoms
b) Attachment of oxygenated groups on the sp2 hybridized surfaces
c) Doping graphene with non-carbon atoms
d) Removal of defects from the graphene sheets
View Answer
Explanation: Defect free graphene sheets appear to be chemically inert. Introduction of defects in the graphene, doping graphene sheets with non-carbon atoms, like nitrogen, can enhance its chemical reactivity. Moreover, reaction of graphene with halogens like chlorine, fluorine raises chemical reactivity. Finally the attachment of oxygenated groups on the sp2 hybridized surfaces of graphene enhances their hydrophilicity and reactivity.
5. Choose the correct pair of properties shown by crumpled graphene.
a) Super-capacitance and super-hydrophilicity
b) Super-hydrophobicity and tunable wettability
c) Super-hydrophilicity and tunable wettability
d) Inferior conductivity and high specific surface area
View Answer
Explanation: Defects when introduced into graphene sheets can increase their chemical reactivity to a great extent. Hence, highly crumpled graphene samples show super-hydrophobicity and tunable wettability. Apart from that they also exhibit super capacitance due to their unique features like extremely high specific surface area, superior conductivity etc.
6. Nitrogen-doped graphene is an efficient electrocatalyst for various reduction processes.
a) True
b) False
View Answer
Explanation: Graphene when doped with nitrogen can act as electrocatalyst for various reduction processes. For instance, selective reduction of carbon dioxide (CO2) to formate in aqueous solution is performed using metal free nitrogen doped graphene.
7. Which of the following option regarding graphene edges correctly represent the given diagram?
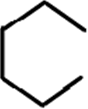
a) Bay
b) Gulf
c) Fjord
d) Cove
View Answer
Explanation: Graphene shows a number of edge topologies.
Cove is represented as:
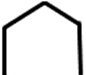
Bay is represented as:
Gulf is represented as:
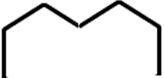
8. Which of the following is not a Graphene edge topology?
a) Chiral
b) Bay
c) Fjord
d) Cove
View Answer
Explanation: Graphene has several edge topologies. These mainly include bay, fjord, cove, zigzag, gulf, armchair and k-region. Out of these, zigzag and armchair are the two main types of edges along the crystallographic directions in graphene.
9. Which of the following options is not one of the reasons for the weak chemical reactivity of graphene basal plane?
a) Absence of dangling bonds
b) Less curvature of structure
c) Presence of dangling bonds
d) Giant π-conjugation system
View Answer
Explanation: The chemical reactivity of graphene basal plan is weaker than the edges. This is because the basal plane has giant π-conjugation system, less curvature of structure and the absence of dangling bonds.
10. Single-layer graphene is chemically more reactive than thick layer sheets.
a) False
b) True
View Answer
Explanation: Graphene is the only form of carbon in which every carbon atom from the two sides of the sheet is able to react chemically. This is because of the 2D structure of graphene single-layer sheets. Research has also revealed that single layer graphene sheet is 100 times more chemically reactive than multiple layer sheets.
Sanfoundry Global Education & Learning Series – Nanotechnology.
To practice all areas of Nanotechnology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
