This set of Engineering Hydrology Multiple Choice Questions & Answers (MCQs) focuses on “Evaporation Process – Set 2”.
1. Which of the following graphs represents the correct relationship between wind velocity and evaporation rate?
a) 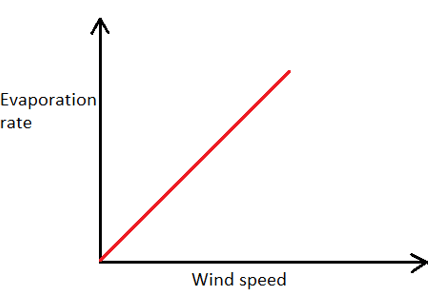
b) 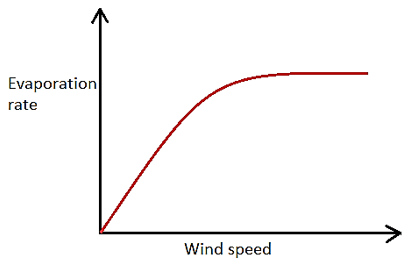
c) 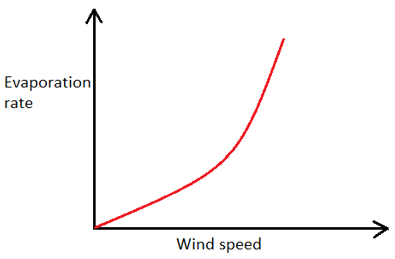
d) 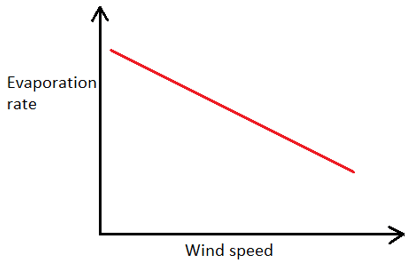
View Answer
Explanation: As the velocity of wind blowing over a water body increases, the rate of evaporation also increases, but upto a certain value of wind velocity after which any further increase in wind velocity does not affect the rate of evaporation
2. The critical wind velocity depends on which of the following?
a) Temperature of water body
b) Shape of water body
c) Size of water body
d) Depth of water body
View Answer
Explanation: Since the wind blows over the surface of the water body, larger bodies will require stronger winds to cause the same rate of evaporation compared to smaller water bodies. Which implies, the rate of evaporation will reach a constant value at lower critical wind speeds for smaller water bodies, and at higher wind speeds for larger water bodies.
3. As the humidity increases, the rate of evaporation increases.
a) True
b) False
View Answer
Explanation: Humidity basically implies the amount of water vapour present in the air relative to the saturation capacity. As the humidity, or relative vapour pressure increases, the ability of the air to hold more vapours decreases. Therefore, this results in a decrease in the rate of evaporation.
4. There are four similar sized ponds A, B,C and D in a meteorologically homogeneous region. The following table gives the altitudes of the surfaces of these ponds with respect to MSL.
| Ponds | A | B | C | D |
| Surface altitude above MSL (in m) | 1522 | 877 | 1035 | 1784 |
Which of the following ponds is most likely experience the highest evaporation in a given year?
a) A
b) B
c) C
d) D
View Answer
Explanation: As we go higher up in altitude, the atmospheric pressure decreases. This means that water molecules will have to expend lesser energy to evaporate compared to ponds at lower altitudes where the air pressure near the surface will be higher. Since pond D is located at the highest altitude, it will most likely undergo the highest evaporation.
5. Four different solutions of equal quantity are kept in an evaporation setup and observed for two weeks. Which of the following solutions will have evaporated the least?
a) 0.01 M of NaCl
b) 0.1 M of NaCl
c) 1 M of NaCl
d) 1 M of Na2SO4
View Answer
Explanation: Out of the two 1 M solutions, the sodium sulphate solution has lesser vapour pressure as it dissociates into 3 ions whereas the sodium chloride solution dissociates into 2. Since 1 M of Na2SO4 has the least vapour pressure of all the solutions, it will also have the lowest rate of evaporation.
6. One litre of freshwater and one litre of sea water is collected separately in two different pans and are subjected to evaporation under identical conditions. After the study period, it was observed that 500 ml of water was left in the freshwater pan. What is the likely amount of water to be left in the seawater pan?
a) 500 ml
b) >500 ml
c) <500 ml
d) Cannot be determined
View Answer
Explanation: Since seawater contains dissolves salts, it has a lower rate of evaporation than freshwater. Since 500 ml of freshwater had evaporated, it can be expected that less than 500 ml of seawater would evaporate. Therefore, the amount of seawater left in the pan must be more than 500 ml.
7. Two beakers A and B are filled with 500 ml of freshwater and 500 ml of seawater, respectively. Both the beakers were kept for evaporation for one week. During the observation, beaker B broke and the amount of water left in beaker A was noted to be 389 ml. Due to time constraints, the experiment cannot be repeated. Which of the following readings would most likely depict the reading of the leftover water in beaker B at the end of one week?
a) 392 ml
b) 431 ml
c) 336 ml
d) 380 ml
View Answer
Explanation: Generally, the evaporation from seawater is 2-3% less than the evaporation from freshwater under identical conditions. Which implies that the beaker B should have more water left than that in beaker A. So the reading cannot be 336 ml or 380 ml. Also, the amount of freshwater that had evaporated is 500 – 389 = 111 ml. Now,
Case 1: 431 ml
Water evaporated = 500 – 431 = 69 ml
→ % less than freshwater evaporation = \(\frac{111-69}{111}\)*100=37.84% >> 2 to 3%
Case 2: Case 2: 392 ml
Water evaporated = 500 – 392 = 108 ml
→ % less than freshwater evaporation = \(\frac{111-108}{111}\)*100=2.7%
Therefore, the most likely reading would be 392 ml.
8. The depth of a particular lake changes constantly throughout the summer season as shown in the variation graph. In which month will it experience the least evaporation?
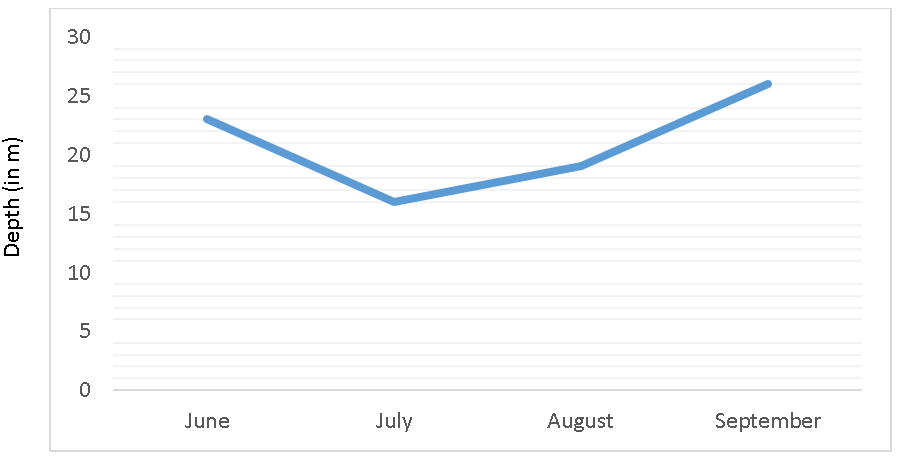
a) June
b) July
c) August
d) September
View Answer
Explanation: During September, the lake has the most depth which means it can store more heat energy during this month causing it to have the least evaporation. The shallower the water depth, the more the evaporation during summer season.
Sanfoundry Global Education & Learning Series – Engineering Hydrology.
To practice all areas of Engineering Hydrology, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
