This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Temperature & Pressure Effects – Equilibrium Conversion”.
1. State true or false.
It is possible to achieve a conversion greater than the equilibrium conversion.
a) True
b) False
View Answer
Explanation: Equilibrium conversion is the maximum value of conversion that can be achieved in any system. Conversion above the equilibrium value of conversion is not possible.
2. State true or false.
A reversible reaction is the one in which the reaction proceeds simultaneously in both directions.
a) True
b) False
View Answer
Explanation: A reversible reaction occurs simultaneously and independently in both directions. It is represented in the general form A ↔ R.
3. For the reaction A ↔ R, which of the following is the correct expression of CR?
a) CR = CA0XA
b) CR = CA0XA
c) CR = CR0 + CA0
d) CR = CR0 + CA0XA
View Answer
Explanation: The final concentration of R is given as CR= CR0 + CA0XA. CA0XA is the amount of A converted to R, which is the amount of R formed.
4. Equilibrium is a state in which ___
a) Rate of forward reaction > Rate of backward reaction
b) Rate of forward reaction = Rate of backward reaction
c) Rate of forward reaction < Rate of backward reaction
d) Rate of forward reaction is independent of the rate of backward reaction
View Answer
Explanation: At equilibrium, the net rate of reaction is zero. The rate of change of concentration of A is zero.
5. For the reaction A ↔ R, k1 being the rate constant of forward reaction, k2 being the rate constant of backward reaction and XAe is the equilibrium conversion, k2 is obtained as _____
a) k2 = k1\(\frac{(1 – X_{Ae})}{(\frac{C_{A0}}{C_{R0}} + X_{Ae})} \)
b) k2 = k1\(\frac{(1 + X_{Ae})}{(\frac{C_{A0}}{C_{R0}} + X_{Ae})} \)
c) k2 = k1\(\frac{(1 – X_{Ae})}{(\frac{C_{A0}}{C_{R0}} – X_{Ae})} \)
d) k2 = k1\(\frac{(1 – X_{Ae})}{(\frac{C_{A0}}{C_{R0}} – X_{Ae})} \)
View Answer
Explanation: At equilibrium, rate is zero. Hence, k2(\(\frac{C_{A0}}{C_{R0}}\) + XAe) – k1(1- XAe) = 0. k2 = k1\(\frac{(1 – X_{Ae})}{(\frac{C_{A0}}{C_{R0}} + X_{Ae})}. \)
6. The plot representing the relation of equilibrium conversion to the reaction time for a unimolecular first order reaction is ____
a) 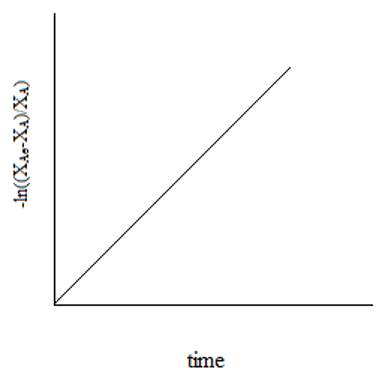
b) 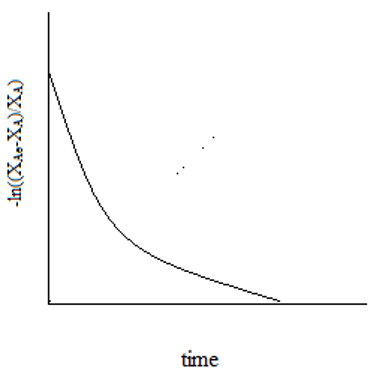
c) 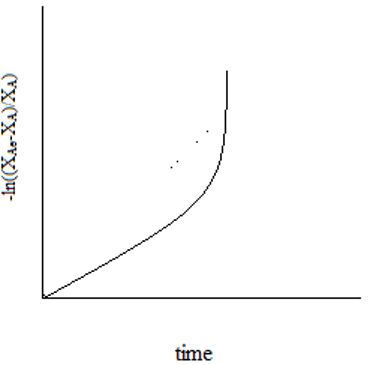
d) 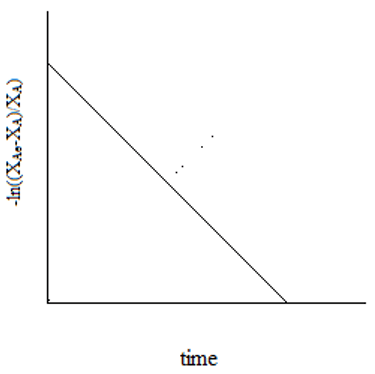
View Answer
Explanation: The equation representing the first order reaction is -ln\((\frac{X_{Ae}-X_A)}{X_{Ae}})\) = k1t \(\frac{(1+\frac{C_{A0}}{C_{R0}})}{(\frac{C_{A0}}{C_{R0}} + X_{Ae})}.\) The equation represents a straight line passing through the origin.
7. Which of the following represents second order bimolecular type reversible reaction?
a) A+B ↔ R+S
b) A ↔ B
c) A ↔ R+S
d) A+B ↔ R
View Answer
Explanation: Bimolecular reaction involves 2 molecules. Both reactants and products react simultaneously.
8. The plot of the function of equilibrium conversion to time for a bimolecular type reversible reaction is ____
a) Exponential
b) Linear
c) Logarithmic
d) Quadratic
View Answer
Explanation: The kinetics is represented as, ln\((\frac{X_{Ae}-2(X_{Ae}-1)X_A}{X_{Ae}-X_A})\) = 2k1\((\frac{1}{X_{Ae}}-1)\)CA0t. This represents the equation of a straight line.
9. Which of the following is not a reversible reaction?
a) Ammonia formation from its elements
b) Formation of hydrogen bromide from its elements
c) Decomposition reaction of nitrogen pentoxide
d) Oxidation of sulphur dioxide to sulphur trioxide
View Answer
Explanation: The reaction of hydrogen with bromine is irreversible reaction. H2 + Br2 → HBr.
10. The Le- Chatlier principle states that ____
a) No reaction is reversible
b) All reactions are reversible at high temperatures
c) A system experiencing disturbance shifts direction of equilibrium to nullify the change
d) A system experiencing disturbance does not shift direction of equilibrium to nullify the change
View Answer
Explanation: For a reversible reaction, any disturbance in the form of variation in temperature, pressure or concentration affects the equilibrium state. The equilibrium shifts in a direction such that the effect is counteracted.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Biotechnology Internship
- Check Biotechnology Books
- Check Chemical Reaction Engineering Books
