This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “ Microbial Fermentation – 1”.
1. State true or false.
Kinetics of microbial fermentation cannot be expressed by Monod kinetics.
a) True
b) False
View Answer
Explanation: The growth rate of microbial cells is given by Monod kinetics.
Rate of growth rC = \(\frac{kC_A C_C}{C_A+ C_M}.\) The reaction is A \(^{\underrightarrow{C}}\) C + R. The substrate in the presence of microbial cells gives more cells and the waste, R.
2. The plot representing the slope of growth curve and CA is ____
a) 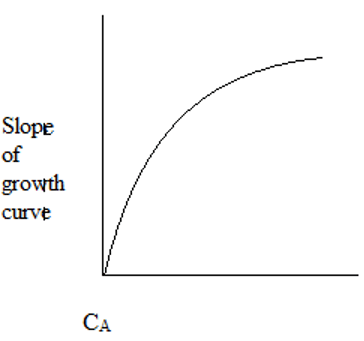
b) 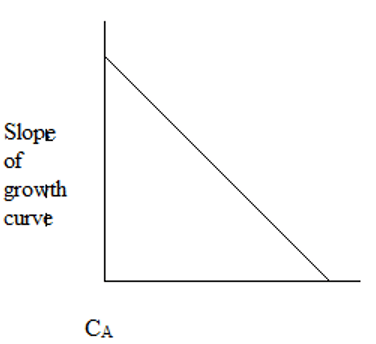
c) 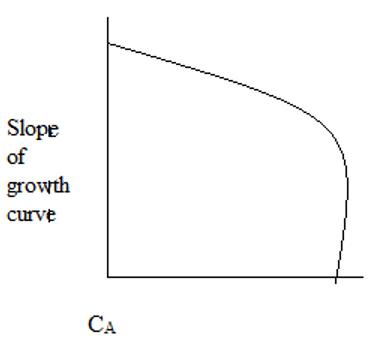
d) 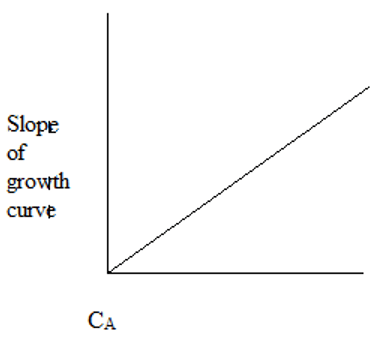
View Answer
Explanation: Initially, the cells starve for food due to low CA. As CA increases, the cells have plenty of food and maximum growth rate is observed.
3. The hierarchy of phases for the microbes in a fermentation process is ____
a) Exponential growth phase → Lag phase → Stationary phase → Death phase
b) Stationary phase → Lag phase → Exponential growth phase → Death phase
c) Lag phase → Exponential growth phase → Stationary phase → Death phase
d) Lag phase → Stationary phase → Exponential growth phase → Death phase
View Answer
Explanation: There is an initial lag phase for the microbial cells to begin growth and reproduction. The cells then grow exponentially and there exists a stationary growth less phase. Eventual death of cells is observed due to depletion of food.
4. The substrate limiting kinetics of A \(^{\underrightarrow{C}}\) C + R is the one in which ___
a) Final concentration of C depends on initial amount of food
b) Final concentration of C does not depend on initial amount of food
c) Final concentration of C does not exceed a certain value
d) The rate depends on enzyme concentration
View Answer
Explanation: The amount of food determines the progress of the reaction. Product does not affect the reaction rate.
5. The poison limiting kinetics of A \(^{\underrightarrow{C}}\) C + R is the one in which ___
a) Final concentration of C depends on initial amount of food
b) Final concentration of C does not depend on initial amount of food
c) Final concentration of C does not exceed a certain value
d) The rate depends on enzyme concentration
View Answer
Explanation: The product slows down the reaction. After sometime, the product concentration increases and the reaction cease to occur.
6. The plot representing the maximum final cell concentration and initial concentration of food in a batch fermenter is ____
a)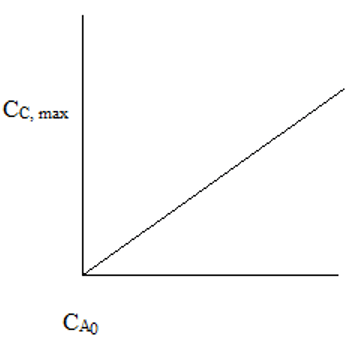
b) 
c) 
d) 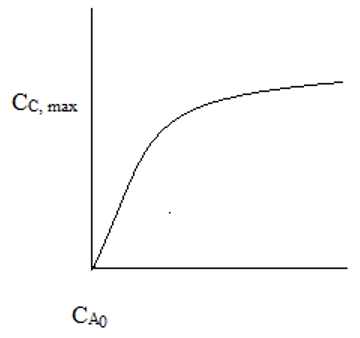
View Answer
Explanation: At low CA0, the reaction is substrate limiting. At high CA0, the reaction is poison limiting. CC,max is independent of CA0 at high CA0.
7. In a batch fermenter, for substrate limiting kinetics, CC,max is ________
a) Independent of CA0
b) Directly proportional to CA0
c) Inversely proportional to CA0
d) Proportional to the square of CA0
View Answer
Explanation: At low CA0, CC,max α CA0. For high CA0, CC,max is independent of CA0.
8. With an accumulation of waste formed during the reaction, the value of observed Monod rate constant ____
a) Decreases
b) Increases
c) Remains constant
d) First decreases and then increases
View Answer
Explanation: The accumulation of waste formed decelerates the reaction rate. This decrease is reflected in the reaction rate constant.
9. The relationship between the Monod rate constant, k and the rate constant observed due to waste accumulation kobs is ____
a) kobs = k (1-\(\frac{C_R}{C_R^*}\))n
b) kobs = k (1-\(\frac{C_R}{C_R^*})^\frac{1}{n}\)
c) k = kobs (1-\(\frac{C_R}{C_R^*}\))n
d) kobs = k (\(\frac{C_R}{C_R^*}\))n
CR* is the concentration of R where cell activity stops and n is the order of product poisoning.
View Answer
Explanation: kobs = k (1-\(\frac{C_R}{C_R^*}\))n
The buildup of harmful wastes interferes with the cell multiplication. The observed rate constant decreases with a rise in CR.
10. The fractional yield of C with respect to A for the reaction A \(^{\underrightarrow{C}}\) cC + rR is the ratio of ____
a) All R formed to all A reacted
b) All A reacted to all R formed
c) All A reacted to all C formed
d) All C formed to all A reacted
View Answer
Explanation: Fractional yield is the ratio of product formed to the total reactant fed. It is the ratio of all C formed to all A reacted.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Biotechnology Internship
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Chemical Reaction Engineering Books
- Practice Biotechnology MCQs
