This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Design of CSTRS in Series”.
1. State true or false.
It is preferable to use a number of CSTRs in series to achieve a specific conversion than a single CSTR.
a) True
b) False
View Answer
Explanation: Total volume required to achieve a given conversion is less than the volume of a single CSTR. A number of CSTRs in series approaches Plug Flow behavior.
2. What is the mole balance of a component in a CSTR? (Where F0 is the molar flowrate of reactant entering and F is the exit molar flowrate)
a) F0 = FX + F
b) F0 = FX + F0
c) F0 = F0X + F
d) F0 = X + F
View Answer
Explanation: The mole balance of a component in a CSTR is given as F0 = F0X + F. Molar flowrate of a component A fed to the reactor is the sum of molar flowrate at which A is consumed and molar flowrate at which A leaves the reactor.
3. For ‘i’ CSTRs of variable volume in series, the residence time in the ith reactor is ____
a) τi = \(\frac{- C_i}{-r_i} \)
b) τi = \(\frac{C_{i-1}- C_i}{-r_i} \)
c) τi = \(\frac{C_i}{-r_i} \)
d) τi = \(\frac{C_{i-1}}{-r_i} \)
View Answer
Explanation: For 2nd reactor in series, the mole balance in 2nd reactor is vC1 = vC2 + (-r2)V2.
v is the volumetric flowrate and V2 is the volume of the 2nd reactor. Rearranging, \(\frac{V_2}{v} = \frac{C_1- C_2}{-r_2}\) , τ2 = \(\frac{C_1- C_2}{-r_2}.\) For ith reactor in series, τi = \(\frac{C_{i-1}- C_i}{-r_i}. \)
4. What is the concentration C4 at the end of 4th reactor, for n unequal sized CSTRs in series, where X4 is the conversion in 4th reactor?
a) C4 = (1 – X4)
b) C4 = C0X4
c) C0 = C4 (1 – X4)
d) C4 = C0(1 – X4)
View Answer
Explanation: Conversion in the 4th reactor is the ratio of reactant converted in the 4th reactor to the initial concentration. X4 = \(\frac{C_0 – C_4}{C_0}.\) Hence, C4 = C0(1 – X4).
5. The plot of rate of the reaction and the reactant concentration for unequal sized CSTRs in series is ____
a) 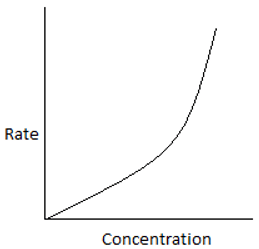
b) 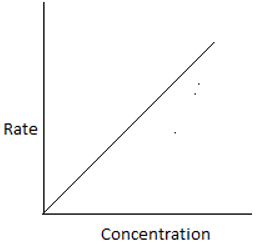
c) 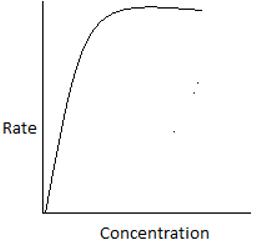
d) 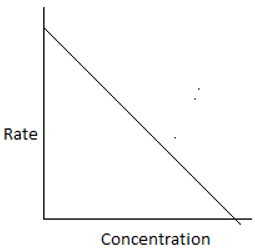
View Answer
Explanation:For ith reactor, \(\frac{-1}{-τ_i} = \frac{-r_i}{C_i-C_{i-1}}.\) For the 1st reactor in series, the rate is high and decreases for every consecutive reactor. Hence, the plot shows a high rate for the initial concentration.
6. For equal sized CSTRs in series carrying out 1st order reactions of constant density, which of the following is true? (ki is the rate constant in a reactor ‘i’ in series)
a) k1 ≠ k2 ≠ … ≠ kn, τ1 = τ2 = … = τn
b) k1 = k2 = … = kn, τ1 = τ2 = … = τn
c) k1 = k2 = … = kn, τ1 ≠ τ2 ≠ … ≠ τn
d) k1< k2< …<kn, τ1 = τ2 = … = τn
View Answer
Explanation: All the CSTRs are operated at the same temperature. Hence, k1 = k2 = … = kn. The volume of all the CSTRs is the same, V1 = V2 = … = Vn. τ = \(\frac{V}{v_0},\) hence τ1 = τ2 = … = τn.
7. For very low conversion, the value of Damkohler number is ____
a) ≤0.1
b) >10
c) ≥0.1
d) = 10
View Answer
Explanation: Damkohler number estimates the conversion that can be achieved in a flow reactor. At low conversions nearly 10%, Da≤0.1
8. State true or false.
For different sized CSTRs in series, the best system is the one having a minimum volume for a specific conversion.
a) True
b) False
View Answer
Explanation: The optimum size ratio of CSTRs in series depends on the reaction kinetics. For a first order reaction, equal sized CSTRs are operated. For positive order reactions, smaller CSTR is followed by larger ones.
9. For equal sized CSTRs in series carrying out 1st order reaction, the concentration at the outlet of nth reactor is ____
a) CN = \(\frac{1}{(1 + τk)^N} \)
b) CN = \(\frac{C_0}{(1 + τ)^N} \)
c) CN = \(\frac{C_0}{(1 + k)^N} \)
d) CN = \(\frac{C_0}{(1 + τk)^N} \)
View Answer
Explanation: τ1 = \(\frac{C_0- C_1}{-r_1} = \frac{C_0- C_1}{kC_1}.\) Rearranging, C1 = \(\frac{C_0}{(1+ τk)}.\) In 2nd reactor, τ2 = \(\frac{C_1- C_2}{kC_2}.\) Similarly, for nth reactor, CN = \(\frac{C_0}{(1 + τk)^N}.\)
10. If Da is the Damkohler number, for equal sized CSTRs in series carrying out first order reaction, the plot representing conversion and number of CSTRs is ____
a) 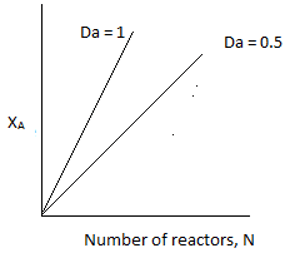
b) 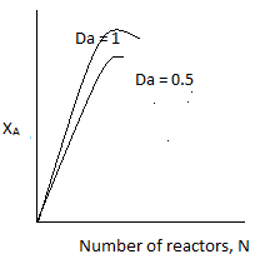
c) 
d) 
View Answer
Explanation: At kτ ≥ 1, nearly 90% conversion is achieved. Such a high conversion is achieved in 2 or three consecutive reactors.At kτ<1, conversion increases significantly with the addition of every reactor.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Reaction Engineering Books
- Apply for Chemical Engineering Internship
- Apply for Biotechnology Internship
- Practice Chemical Engineering MCQs
- Check Biotechnology Books
