This set of Class 11 Chemistry Chapter 7 Multiple Choice Questions & Answers (MCQs) focuses on “Equilibrium in Chemical Processes – Dynamic Equilibrium”.
1. What does the dotted curve in the diagram given below represent?
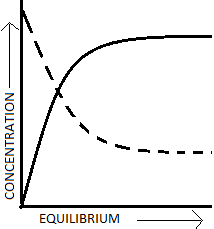
a) concentration of reactants
b) equilibrium constant
c) concentration of products
d) concentration of catalyst
View Answer
Explanation: The dotted curve in the above diagram the concentration of products as it is increasing with time and the attainment of equilibrium, the Strait curve is a concentration of reactants as it is decreasing as the reaction proceeds.
2. Synthesis of ammonia occurs through __________
a) Haber’s process
b) The carbon cycle
c) Nitrogen cycle
d) Hydrogen cycle
View Answer
Explanation: Haber’s process allows nitrogen from the air to combine with hydrogen in order to form ammonia, this process is reversible and exothermic. This process occurs at high temperatures and a catalyst is a form of iron.
3. Equilibrium can be attained only from one side.
a) true
b) false
View Answer
Explanation: An equilibrium can be obtained from both sides this is because a chemical reaction that reaches a state of dynamic equilibrium in which there are equal rates of forwarding and backward reactions and there is no net change in composition.
4. At dynamic equilibrium the concentration of both the reactants and products are ____________
a) equal
b) not equal
c) cannot predict
d) sometimes equal sometimes not equal
View Answer
Explanation: A dynamic equilibrium in a chemical reaction, both forward and backward rates are always equal. As the reaction occurs the concentration of product increases and the concentration of reactants decrease at equilibrium they are not equal.
5. In which of the following conditions do you think the rates of both forward and backward reactions are the same?
a) unstable equilibrium
b) not in an equilibrium
c) the beginning of a reaction
d) equilibrium
View Answer
Explanation: In a chemical reaction a state comes when both the forward and reverse reactions occur at the same rate and this state is known as equilibrium. At the beginning of the reaction, the rate of the Forward reaction is higher than the rate of backward reaction.
6. What is the reverse reaction of s of water?
a) conversion into ice
b) conversion into steam
c) conversion into water
d) equilibria
View Answer
Explanation: The process of solidifying of water is nothing but the formation of ice that is a condensation of water the reverse reaction of condensation of water is the melting of water, that is the formation of water from ice.
7. Dynamic equilibrium mainly concerns about _______________
a) spontaneous reactions
b) nonspontaneous reactions
c) reversible reactions
d) Irreversible reactions
View Answer
Explanation: In case of dynamic equilibrium, the ratio of reactants and products changes. The net change equals zero, as there is no net movement of particles. The formation of the reactants is equal to the formation of products here in dynamic equilibrium.
8. At which of the following temperatures water is a dynamic equilibrium with ice?
a) 0 Kelvin
b) zero degree centigrade
c) zero degree Fahrenheit
d) 100 Kelvin
View Answer
Explanation: Water is a dynamic equilibrium with ice at the freezing point of water that is zero degrees centigrade, 273 Kelvin and 32-degree Fahrenheit. Because at zero degrees centigrade the phase transition occurs.
9. When a chemical reaction is written the products are written on the _____________
a) left side
b) right side
c) either left side or right side
d) depends on the chemicals present
View Answer
Explanation: When a chemical reaction is written, the reactants are written on the left side and the products are written on the right side, while both parts of the reaction are joined by an arrow mark (→).
10. Equilibrium of a reaction depends on the concentration of reactants.
a) true
b) false
View Answer
Explanation: In a chemical reaction equilibrium does not depend on the concentration of reactants as well as a concentration of products. Equilibrium is attained when the rates of both forward and backward reactions are equal.
Sanfoundry Global Education & Learning Series – Chemistry – Class 11.
To practice all chapters and topics of class 11 Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Chemistry MCQs
- Practice Class 11 - Physics MCQs
- Check Class 11 - Chemistry Books
- Practice Class 11 - Mathematics MCQs
- Practice Class 11 - Biology MCQs
