This set of Chemical Reaction Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Reaction Rate Constant and Activation Energy”.
1. Which of these, represents the dimension of rate constant of a first order chemical reaction?
a) (time)-1
b) (concentration) (time)-1
c) (concentration)2 (time)-1
d) (concentration)-1 (time)-1
View Answer
Explanation: The dimension of rate constant is given by (concentration)1-n (time)-1. For first order reaction, n = 1, resulting the concentration term be equal to 1. Thus, the dimension of the rate constant equals to the inverse of time.
2. The rate constant for elementary reactions, according to Arrhenius’ law depends on which factor?
a) Composition
b) Composition and temperature
c) Temperature
d) Depends on reactant
View Answer
Explanation: The Arrhenius law is given by the equation k = k0 e– E / RT, where k0 is the pre – exponential factor and E is the activation energy of the reaction, R is the universal gas constant and T corresponds to temperature. By observation we can easily understand that the rate constant depends on the temperature.
3. For a batch reactor, the batch time is 30 min at 67 degree Celsius to form a product. However, a new technology is found such that the batch reduces to 15 mins, when heated to 77 degree Celsius. Find the activation energy, assuming that activation energy does not change with temperature.
a) 68500 J / mol
b) 68577.62 J / mol
c) 59753.51 J / mol
d) 76458.62 J / mol
View Answer
Explanation: Given that,
t1 = 30 min
t2 = 15 min
T1 = 340 K
T2 = 350 K
Now, according to Arrhenius law,
ln\(\frac {r_2}{r_1}\) = ln\(\frac {t_1}{t_2} = \frac {E}{R} \big [ \frac {1}{T_1} – \frac {1}{T_2} \big ]\)
Substituting the values,
ln\(\frac {30}{15} = \frac {E}{8.314} \big [ \frac {1}{340} – \frac {1}{350} \big ] \)
E = \(\frac {0.6931×8.314×340×350}{(350 – 340)}\)
E = 68577.62 \(\frac {J}{mol}\).
4. A Arrhenius plot of lnk vs 1 / T gives a straight line, which of these represents the vale of slope of the line?
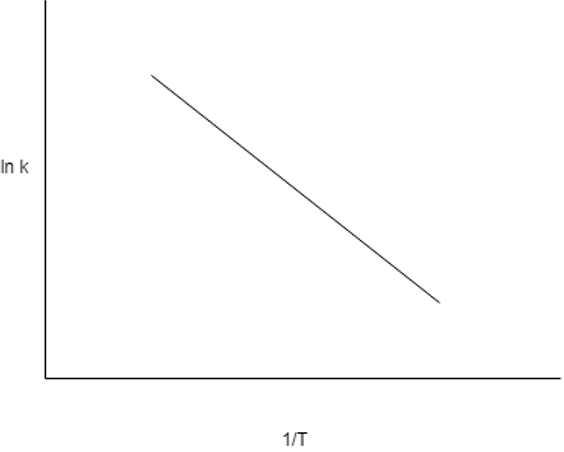
a) E
b) E / T2
c) E / R2
d) E / R
View Answer
Explanation: According to the Arrhenius law, lnk = lnk0 – \(\frac {E }{RT}\) or lnk ∝ –\(\frac {E }{RT}\). Thus, according to the straight – line equation y = mx + c, where m is the slope, we get the slope as E / R. Therefore, for an Arrhenius plot, a large slope indicates large activation energy and vice versa. Reactions with high activation energies are temperature sensitive reactions and the one with low energy are relatively temperature insensitive.
5. Activation energy is the energy lost by the reactants.
a) True
b) False
View Answer
Explanation: Activation energy is the minimum energy (potential) required by the reactants to transform them to products. It can be assumed as a barrier for the reactants to overcome and form products. This potential energy is provided by the kinetic energy of the colliding molecules. The increase in the energy of the system causes them to reach an activated state and allow them to break and form new bonds.
6. Which line represents the highest value of activation energy in the following Arrhenius plot?
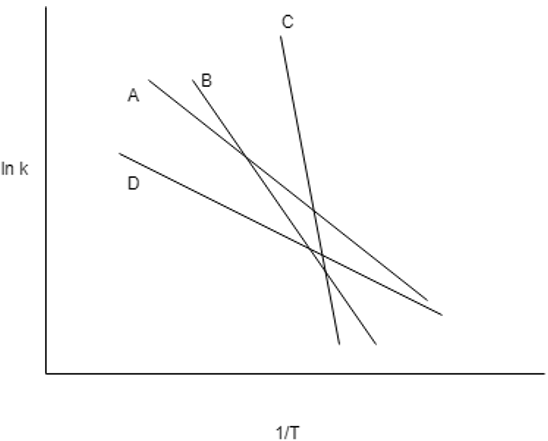
a) A
b) B
c) C
d) D
View Answer
Explanation: From the Arrhenius theory, we know that if the slope of the line is more, the energy of activation is more. Alternatively, system with high energy of activation are more temperature sensitive. For a small change in temperature, the change in the rate constant is large. The Line C has more slope and thus it has large energy of activation.
7. Which line represents the lowest value of activation energy in the following Arrhenius plot?

a) A
b) B
c) C
d) D
View Answer
Explanation: From the Arrhenius theory, we know that if the slope of the line is more, the energy of activation is more. The Line D has less slope than others and thus it has low energy of activation. Systems with low energy of activation are less sensitive to temperature.
8. If the rate of reaction is thrice when the temperature is increased from 300K to 400K, then find the energy of activation. Assume that the energy of activation does not change with change in temperature.
a) 11523.16 J / mol
b) 25486.98 J / mol
c) 5213.57 J / mol
d) 10960.63 J / mol
View Answer
Explanation: Given that,
k2 = 3 k1
T1 = 300 K
T2 = 400 K
Now, according to Arrhenius law,
ln\(\frac {k_2}{k_1} = \frac {E}{R} \big [ \frac {1}{T_1} – \frac {1}{T_2} \big ] \)
Substituting the values,
ln\(\frac {3k_1}{k_1} = \frac {E}{8.314} \big [ \frac {1}{300} – \frac {1}{400} \big ] \)
E = \(\frac {1.0986×8.314×300×400}{(400 – 300)} \)
E = 10960.63 J / mol.
9. For the chemical reaction 2R + S → T, the rates of formation of each species respectively are rR, rS and rT. What is the relation between rR, rS and rT?
a) 2rR = rS = rT
b) 2rR = rS = – rT
c) rR = 2rS = 2rT
d) rR = 2rS = – 2rT
View Answer
For a chemical reaction, aA + bB → cC + dD
The rates of formation are related as \(\frac {- r_A}{a} = \frac {- r_B}{b} = \frac {r_C}{c} = \frac {r_D}{d} \)
So, for the given reaction in the question \(\frac {- r_R}{2} = \frac {- r_S}{1} = \frac {r_T}{1}\) which on rearranging gives rR = 2rS = – 2rT.
10. The rate of ammonia synthesis for the reaction N2 + 3H2 → 2NH3 is given by r = 0.8 PN2 P\(_{H_2}^3\) – 0.6P\(_{NH_3}^2\). If the reaction is represented as 1 / 2N2 + 3 / 2H2 – > NH3, the rate of synthesis will be _________
a) r = 0.8 P\(_{N_2}^{0.5}\) P\(_{H_2}^{1.5}\) – 0.6PNH3
b) r = 0.8 PN2 P\(_{H_2}^3\) – 0.6P\(_{NH_3}^2\)
c) r r = 0.5(0.8 P\(_{N_2}^{0.5}\) P\(_{H_2}^{1.5}\) – 0.6PNH3)
d) r = 0.5(0.8 PN2 P\(_{H_2}^3\) – 0.6P\(_{NH_3}^2\)
View Answer
Explanation: The rate of reactions does not depend on the stoichiometry of the reaction. So, whatever be the stoichiometry, the rate of reaction remains unchanged. Hence the rate will be given by r = 0.8 PN2 P\(_{H_2}^3\) – 0.6P\(_{NH_3}^2\).
11. The rate expression for the reaction A + B → C + D, in term of concentration of A (CA) is given by – rA = \(\frac {K_1 C_A^{\frac {1}{2}}}{1 + K_2 C_A^2}\)
The units of K1 and K2 are respectively __________
a) mol1/2 m-3/2 s-1, mol-2 m6
b) mol-1 m3 s-1, mol1/2 m3/2
c) mol m-3 s-1, mol1/2 m3/2 s-1
d) mol-1 m3 s-1, mol-1/2 m3/2 s-1/2
View Answer
Explanation: The numerator of the rate expression should combinedly have the unit of mol / m3 and the denominator should be dimensionless since 1 is dimensionless.
[K1] = [mol m-3 s-1 ] / [mol m-3]1/2 = mol1/2 m-3/2 s-1,
K2 = [mol m-3]-2 = mol-2 m6
12. Which of the following is not correct regarding the Arrhenius law of rate constant?
a) The rate constant K is proportional to temperature of the reaction
b) K is proportional to exponential of (- E / RT)
c) ln\(\frac {r_2}{r_1} = \frac {E}{R} \big ( \frac {1}{T_1} – \frac {1}{T_2} \big )\)
d) ln\(\frac {t_1}{t_2} = \frac {E}{R} \big ( \frac {1}{T_1} – \frac {1}{T_2} \big )\)
View Answer
Explanation: The rate constant K is proportional to exponential of (- E / RT). K = Ko e-E/RT, Ko is called the pre – exponential factor. Comparing two reaction rates r1 and r2 and reaction times t1 and t2, we obtain the two respective expressions: ln\(\frac {r_2}{r_1} = \frac {E}{R} \big ( \frac {1}{T_1} – \frac {1}{T_2} \big )\) and ln\(\frac {t_1}{t_2} = \frac {E}{R} \big ( \frac {1}{T_1} – \frac {1}{T_2} \big )\).
13. Which of the following are correct?
i) Collision theory is given by k α T1/2 e-E/RT. ii) It is valid for unimolecular reactions iii) It is based on the successful collisions of the reacting molecules
a) Only i)
b) Only ii)
c) ii) & iii)
d) i) & iii)
View Answer
Explanation: The collision theory is given by the expression, k α T1/2 e-E/RT. It is invalid for unimolecular reactions because for collision at least two reacting molecules must be present. It is hence based on successful collisions.
14. The general equation for the reaction rate constant is given by
k α Tn e-E/RT
Which is correct?
a) When n = 0, it gives Arrhenius Theory
b) When n = 1 / 2, it gives Collision Theory
c) When n = 1, it gives Transition Theory
d) Nothing can be deduced
View Answer
Explanation:
Arrhenius Theory: k α e-E/RT
Collision Theory: k α T1/2 e-E/RT
Transition Theory: k α T1 e-E/RT
15. For a chemical reaction, the ratio of rate constant at 500K to that at 400K is 1.5. Given R = 8.314 J / mol – K, the value of activation energy is _______
a) 15.24 kJ / mol
b) 6.742 kJ / mol
c) 15.24 J / mol
d) 6742 J / Kmol
View Answer
Explanation: We know Arrhenius Equation for two reaction rate constants at two different temperatures is given by
ln\(\frac {r_2}{r_1} = \frac {E}{R} \big ( \frac {1}{T_1} – \frac {1}{T_2} \big )\)
Given, \(\frac {r_2}{r_1}\) = 1.5. Substituting the values,
ln1.5 = \(\frac {E}{8.314} \big ( \frac {1}{400} – \frac {1}{500} \big )\), E = 6.742 kJ / mol.
16. Which of the following is correct based on Arrhenius model of the rate constant K = Ae-E/RT?
a) A is always dimensionless
b) For two reactions 1 and 2, if A1 = A2 and E1 > E2, then K1(t) > K2(T)
c) For a given reaction, the percentage change of k with respect to temperature is higher at lower temperatures
d) The percentage change of K with respect to temperature is higher for higher A
View Answer
Explanation: As evident from the expression K = Ae-E/RT, A cannot be always dimensionless, it depends on the unit of K. If A1 = A2 and E1 > E2, then K1(t) < K2(T). For lower temperatures, the percentage change of K with respect to temperature is higher.
17. The unit of reaction rate constant (K) for a first order reaction is __________
a) mol m-3 s-1
b) s-1
c) mol-1 m-3 s-1
d) mol s-1
View Answer
Explanation: For first order reaction, – r = KCA and unit of rate of reaction is (- r) is mol m-3 s-1. Using dimensional analysis, we can find the unit of K which is [K] = [s]-1.
18. Arrhenius model has been studied under two conditions. Which of the following is true?
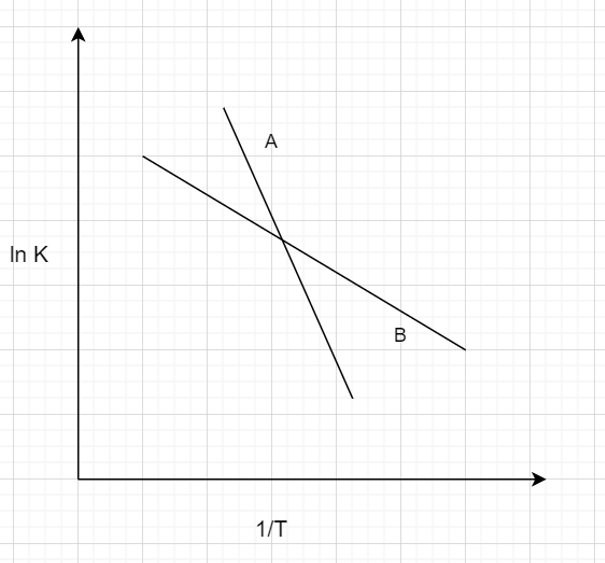
i) A represents more temperature sensitive reaction ii) B represents more temperature sensitive reaction
a) Only i)
b) Only ii)
c) i) & ii)
d) Nothing can be said
View Answer
Explanation: As the Arrhenius model says, k α e-E/RT. The linear curve with high slope is steeper. The steeper the curve, the more is the Activation energy (E), which means the reaction is more dependent on temperature.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Practice Chemical Engineering MCQs
