This set of Chemical Reaction Engineering MCQs focuses on “Choosing of Right Reactor Series or Connections (Maximum Profitability)”.
1. How should the two MFR’s of unequal size be connected in series to maximize production for a first order homogenous reaction?
a) Smaller MFR followed by larger MFR
b) Larger MFR followed by smaller MFR
c) Any arrangement
d) MFR’s should not be connected in series
View Answer
Explanation: The final concentration for both the cases is same and can be calculated using the formula
CA2 = \(\frac{CA0}{(τ1K+1)(τ2k+1)} \)
Hence, it can be inferred that for first order reactions, irrespective of the arrangements the final conversions are going to be the same.
2. For all positive reaction orders and any particular duty the MFR is always larger than the PFR.
a) True
b) False
View Answer
Explanation: Comparison of performance of single MFR and PFR are made for the nth order and was inferred that the MFR is always larger than a PFR for a given duty.
3. How should a PFR and MFR be connected to maximize production from a first order reaction?
a) MFR followed by PFR
b) PFR followed by MFR
c) Any arrangement
d) MFR and PFR should not be connected
View Answer
Explanation: The final concentration for both the cases is same and can be calculated using the formula
CA2 = \(\frac{CA0 e^{-kτp}}{(τmk+1)} \)
Therefore, any arrangement does not make any difference for first order reactions.
4. Graphical procedure for finding conversion in MFR’s of different sizes in series is called ______ method.
a) Newton’s
b) Wheeler’s
c) Octaves
d) Jones
View Answer
Explanation: A graphical procedure was presented by Jones in 1951 to estimate the outlet composition of MFR’s in series.
5. Best arrangement of reactors for a reaction whose rate-concentration curve that rises monotonically.
a) Plug, Large MFR, Small MFR
b) Plug, Small MFR, Large MFR
c) Large MFR, Small MFR, Plug
d) Small MFR, Plug, Large MFR
View Answer
Explanation: For this kind of rate-concentration curve, the concentration should be kept as high as possible, therefore this arrangement should be used.
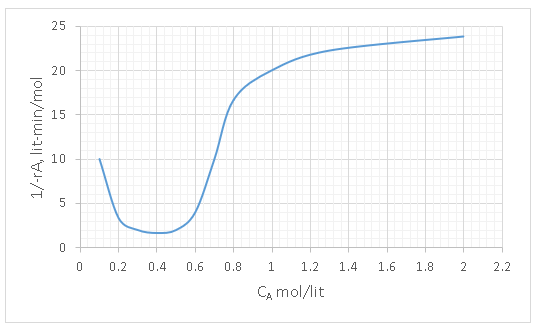
6. Determine the space time of a PFR for the given reaction-concentration curve where the concentration is to be reduced from 1.6 to 0.4 mol/lit in minutes.
a) 5
b) 9
c) 19
d) 32
View Answer
Explanation: The basic design equation for a PFR is
τ = \(\int_{CA0}^{CAf}\frac{-dCA}{-rA} \)
Therefore, the area under the curve in a 1/-rA vs CA gives the space time.
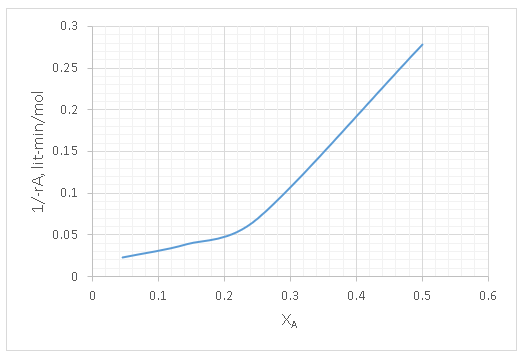
7. Find the size of the MFR in litres required to achieve 40% conversion for a flowrate of 100 lit/min.
a) 10.68
b) 15.83
c) 3.45
d) 7.65
View Answer
Explanation: The basic design equation for a MFR is
\(\frac{τ}{CA0} = \frac{XA}{-rA} \)
A rectangle is to be drawn as shown in the fig below. And the area of the rectangle gives the space time.
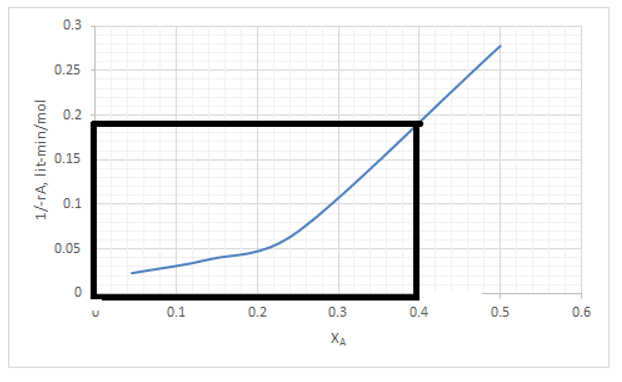
τ = \(\frac{V}{v0} \)
From graph space time was found to be 0.0765 minutes.
So V = 0.0765*100 = 7.65 lit.
8. Four MFR’s are operated in series the total space time is 5 minutes. If it is replaced by a single PFR, What will be the space time? First order reaction with rate constant 2 min-1, concentration reduces from 50 to 10 mol/m3.
a) 0.805
b) 0.123
c) 0562
d) 0.439
View Answer
Explanation: The design equation of PFR is τ = \(\int_{CA0}^{CAf}\frac{-dCA}{-rA} \)
For the 1st order reaction the equation is simplified as kτ = -ln(\(\frac{CA}{CA0}\))
τ = -0.5ln(\(\frac{10}{50}\)) = 0.805 minutes.
9. Effect of flow type on reactor performance when the conversion is small.
a) no effect
b) cannot be determined
c) very small
d) very large
View Answer
Explanation: The reactor performance is not affected by flow type when the conversion is small. If the conversion is very high, flow type largely affects the reactor performance.
10. The concentration profile shown is for __________
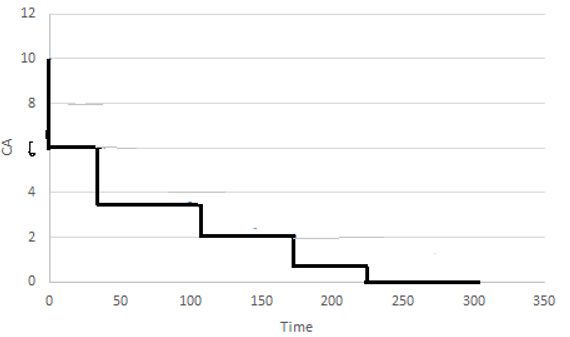
a) MFR in series
b) MFR
c) PFR in series
d) PFR
View Answer
Explanation: The concentration profile is for MFR’s in series. The entire series can be replaced by a PFR whose volume is smaller than the total volume of the MFR’s.
Sanfoundry Global Education & Learning Series – Chemical Reaction Engineering.
To practice MCQs on all areas of Chemical Reaction Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Chemical Reaction Engineering Books
