This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Material Balances -IV”.
1- 4. For the given problem
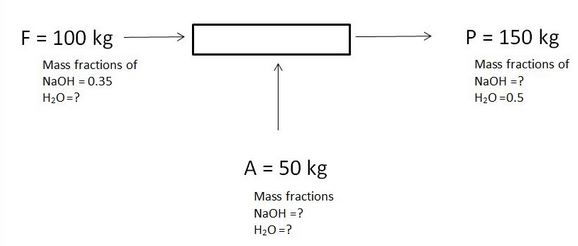
1. What is the mass fraction for H2O in feed and NaOH in product?
a) 0.50 and 0.50
b) 0.65 and 0.50
c) 0.65 and 0.65
d) 0.50 and 0.95
View Answer
Explanation: ΣωI = 1.
2. What is the mass in kg of H2O in feed and NaOH in product?
a) 65 and 50
b) 95 and 50
c) 65 and 75
d) 50 and 50
View Answer
Explanation: ΣωI = 1 and mi= ωIW.
3. What is the mass fraction of NaOH in A?
a) 0.2
b) 0.4
c) 0.6
d) 0.8
View Answer
Explanation: ΣωI = 1 and ΣωIWi = 0 for each component.
4. What is the mass fraction of H2O in A?
a) 0.2
b) 0.4
c) 0.6
d) 0.8
View Answer
Explanation: ΣωI = 1 and ΣωIWi = 0 for each component.
5-7. Tank A contains 90% O2 that is mixed with another tank B containing 30% of O2 to get a Tank C containing 65% O2 .
5. What is the ratio of the gas used from Tank A to that used from Tank B?
a) 1.0
b) 1.2
c) 1.4
d) 1.6
View Answer
Explanation: Σxi = 1.
6. If A = 100 moles, what are the number of moles of B?
a) 51.4
b) 61.4
c) 71.4
d) 81.6
View Answer
Explanation: Σxi = 1.
7. If A = 100 moles, what are the number of moles of C?
a) 151.4
b) 161.4
c) 171.4
d) 181.6
View Answer
Explanation: Σxi = 1.
8. The number of variables whose values are unknown minus the number of independent equation is degree of freedom,
The above given statement is
a) True
b) False
c) It is not the definition of degrees of freedom
d) None of the mentioned
View Answer
Explanation: The number of variables whose values are unknown minus the number of independent equation is degree of freedom.
9. Concept of material balance based upon?
a) Conservation of mass
b) Conservation of energy
c) Conservation of momentum
d) Conservation of Volume
View Answer
Explanation: Material balance is based on conservation of mass.
10. Closed systems have ________ type of boundary wall.
a) Impermeable
b) Permeable
c) Rigid
d) None of the mentioned
View Answer
Explanation: The matter is not allowed to cross the boundary wall of a closed system.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Chemical Process Calculations Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
