This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Element Material Balances”.
1-3. For the given hydro-cracking problem, mole percent of products C4H10, C3H8 and C2H6 are 20.2%, 58.4% and 21.4% respectively.
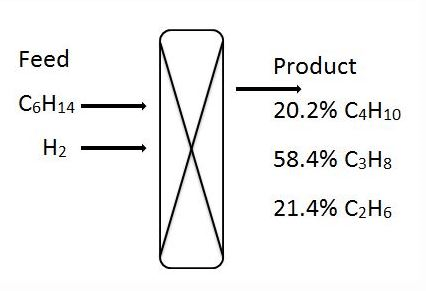
1. What is the mole percent of C6H14 in the feed?
a) 40.2
b) 50.2
c) 60.2
d) 70.2
View Answer
Explanation: Element material balance for each element.
2. What is the mole percent of H2 in the feed?
a) 59.2
b) 49.2
c) 39.2
d) 29.9
View Answer
Explanation: Element material balance for each element.
3. Molar ratio of H2 consumed to Hexane reacted in the process?
a) 1.2
b) 1.4
c) 1.6
d) 1.8
View Answer
Explanation: Element material balance for each element.
4-6. For producing 40 % Methane, 20% Ethane and 40% Butane, a reactor is feed with Pentane and Hydrogen.
4. What is the mole fraction of Hydrogen in the feed?
a) 0.284
b) 0.484
c) 0.684
d) 0.884
View Answer
Explanation: Element material balance for each element.
5. What is the mole fraction of Pentane in the feed?
a) 0.315
b) 0.215
c) 0.115
d) None of the mentioned
View Answer
Explanation: Element material balance for each element.
6. What is the ratio of Hydrogen to the Pentane in the feed?
a) 1.167
b) 2.167
c) 3.167
d) 4.167
View Answer
Explanation: Ratio = 104/48 = 2.167.
7-10. C3H8 is burned in air with a composition of 40 moles and 100 moles.
7. What is the number of moles of CO2 in the product?
a) 12.6
b) 10.6
c) 8.6
d) 4.6
View Answer
Explanation: Moles of CO2 = Extent of the reaction*3.
8. What is the number of moles of H2O in the product?
a) 4.8
b) 8.8
c) 16.8
d) 32.8
View Answer
Explanation: Moles of H2O = Extent of the reaction*2.
9. What is the number of moles of C3H8 in the product?
a) 25.8
b) 35.8
c) 45.8
d) 55.8
View Answer
Explanation: Moles of C3H8 = 40-Extent of the reaction.
10. How many moles of O2 are required for the complete combustion of C3H8?
a) 160.48
b) 170.48
c) 180.48
d) 190.48
View Answer
Explanation: Suppose x moles are required then 0.21x = 48.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Check Chemical Process Calculations Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
