This set of Chemical Process Calculation Multiple Choice Questions & Answers (MCQs) focuses on “Material Balances-I”.
1. Material balance equation can be applied to
a) Total mass
b) Mass of a component
c) Moles of a component
d) All of the mentioned
View Answer
Explanation: Material balance equation can be applied to all these as these are conserved under every change in a chemical process.
2. Material balance equation cannot be applied to
a) Total moles
b) Mass of an atomic species
c) Moles of an atomic species
d) All of the mentioned
View Answer
Explanation: Total moles may vary.
3. We don`t use volume balance in a chemical process because
a) Mass is not conserved in a process
b) Volume is not conserved in a process
c) Both a and b
d) Neither a nor b
View Answer
Explanation: Volume is not conserved in a process because different materials have different densities.
4. In the given tank, there are two feeds and one output. Consider a 2 hour operation; the feed rates are 4000 kg/hr and 6000 kg/hr. The accumulated material inside the tank is 2000 kg. What is the output rate kg/hr of the material?
a) 9000
b) 8000
c) 7000
d) 6000
View Answer
Explanation: Input = output, Take a basis: 2 hour.
5. In a process the inputs and outputs are shown as in the diagram
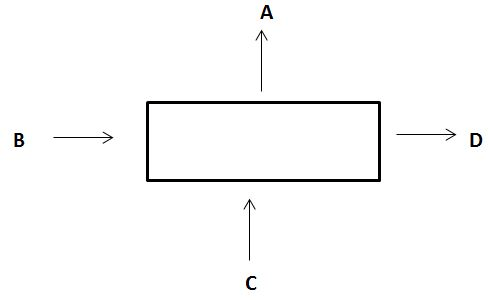
Correct material balance is
a) B+C = A+D
b) A+B = C+D
c) A+C = B+D
d) None of the mentioned
View Answer
Explanation: What comes in, goes out.
6. Based on number of degrees of freedom, find the incorrect statement, consider
NU = Number of unknown
NE = Number of unknown equation
a) NU = NE for that a solution
b) NU > NE > 0 for that more independent equation required
c) NU< NE < 0 for that no solution exists unless some constraints are eliminated
d) None of the mentioned
View Answer
Explanation: ND = NU – NE
Where: ND = Number of degrees of freedom.
7. In a process, two feed streams are there and one output stream. The feed streams have the feed rates as 500 kg/sec and 600 kg/sec. If the output stream rate is 800 kg/s, what is the mass stored in the chamber in five seconds?
a) 300 kg
b) 500 kg
c) 1000 kg
d) 1500 kg
View Answer
Explanation: For five seconds, Accumulation = 5(500+600-800) Kg.
8 – 10. For the given steady state system, consider that a unique solution exists for the process.
ω1, ω2, ω3 are the mass fractions of the components 1, 2 and 3.
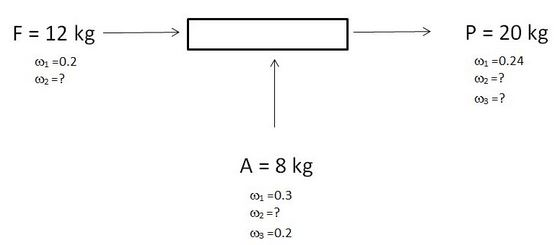
8. What is the value of ω2 for A?
a) 0.5
b) 0.6
c) 0.7
d) 0.8
View Answer
Explanation: Σω = 1.
9. What ids the value of ω2 for P?
a) 0.98
b) 0.88
c) 0.78
d) 0.68
View Answer
Explanation: ω2F + ω2A = ω2P.
10. What ids the value of ω3 for P?
a) 0.09
b) 0.08
c) 0.07
d) 0.06
View Answer
Explanation: ω3A = ω3P.
Sanfoundry Global Education & Learning Series – Chemical Process Calculation.
To practice all areas of Chemical Process Calculation, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Process Calculations Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
