This set of Protein Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Quaternary Structure of Protein”.
1. Which structure of a protein is the arrangement of protein subunits in a multi-subunit complex?
a) Primary
b) Secondary
c) Tertiary
d) Quaternary
View Answer
Explanation: Quaternary structure of a protein is the arrangement of protein subunits in a multi-subunit complex. Many proteins are composed of more than one polypeptide chain. These polypeptide chains fold to form a subunit of a complex protein. All subunits combine to form a complete functional protein.
2. The quaternary structure of a protein is held together by interactions between the side chain groups.
a) True
b) False
View Answer
Explanation: Tertiary structure of a protein consists of secondary structural elements combining to form motifs and domains. Thus, the tertiary structure of a protein is held together by interactions between the side chain groups. Hence, the above statement is false.
3. The Quaternary Structure of a Protein is determined by the gene corresponding to the protein.
a) True
b) False
View Answer
Explanation: The primary structure of a protein is the linear sequence of amino acids in the polypeptide chain. Thus, the primary Structure of a Protein is determined by the gene corresponding to the protein. Hence, the above statement is false.
4. Which of the following options is not suitable for the quaternary structure of a protein?
a) Complex protein
b) More than one polypeptide chain
c) Disulfide bonds
d) Single polypeptide chain
View Answer
Explanation: Generally, complex proteins that have more than one polypeptide chains adopt a quaternary structure. Thus, the option ‘single polypeptide chain’ is not suitable for the quaternary structure. All other options are suitable for the quaternary structure of a protein.
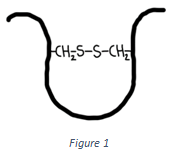
5. Which of the following interactions is shown in the figure below?
a) Hydrogen bond
b) Vander Waals interactions
c) Ionic bond
d) Disulfide bond
View Answer
Explanation: The interaction shown in the above figure is a disulphide bond. Two Cysteine residues are involved in the formation of a disulfide bond. It is covalent in nature.
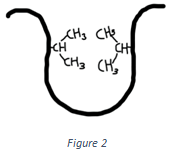
6. Which of the following interactions is shown in the figure below?
a) Disulfide bond
b) Ionic interactions
c) Hydrogen bonds
d) Hydrophobic interactions
View Answer
Explanation: The interaction shown in the above figure is hydrophobic interactions. Hydrophobic interactions involve interaction between two non-polar hydrophobic residues. It is non-covalent in nature.
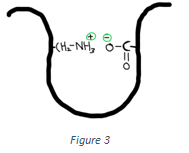
7. Which of the following interactions is shown in the figure below?
a) Disulfide bond
b) Hydrophobic interactions
c) Hydrogen bonds
d) Ionic interactions
View Answer
Explanation: The interaction shown in the above figure is ionic interactions. Ionic interaction is the interaction between two charged groups. If the two charged groups are oppositely charged then they will attract, while two similar charges repel each other.
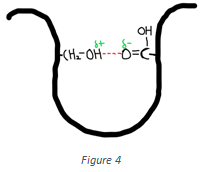
8. Which of the following interactions is shown in the figure below?
a) Disulfide bond
b) Ionic interactions
c) Hydrophobic interactions
d) Hydrogen bonds
View Answer
Explanation: The interaction shown in the above figure is the hydrogen bond.A hydrogen bond is the interaction between a hydrogen atom attached to an electronegative atom with another electronegative atom like oxygen in its vicinity. It is non-covalent in nature.
9. Which type of interactions are involved in the quaternary structure?
a) Only hydrogen bonds
b) Hydrogen bonds and hydrophobic interactions
c) Hydrogen bonds, hydrophobic interactions, and disulfide bonds
d) Hydrogen bonds, hydrophobic interactions, disulfide bonds, and ionic interactions
View Answer
Explanation: Quaternary structure of a protein is made of a combination of any of the following interactions: i) Hydrogen bonds, ii) hydrophobic interactions, iii) disulfide bonds, iv) ionic interactions. Thus, the option containing all of the above options is correct.
10. Which of the protein is not associated with quaternary structure?
a) Insulin
b) Hemoglobin
c) Myoglobin
d) B chain of Insulin
View Answer
Explanation: B chain of Insulin contains only a single polypeptide chain folded into a tertiary structure. Hence, it cannot have a quaternary structure. All other proteins have a quaternary structure.
11. What are multi-subunit proteins called when some or all of its subunits are identical?
a) Monomers
b) Polymers
c) Protomers
d) Oligomers
View Answer
Explanation: Many proteins have a complex structure i.e. they have a multi-subunit structure. When some or all the sub-units of a complex protein are identical it is called oligomers.
12. What are identical subunits called in a multi-subunit protein?
a) Monomers
b) Polymers
c) Oligomers
d) Protomers
View Answer
Explanation: The identical subunits of a multi-subunit protein is called as protomers. An oligomer is when a multi-subunit protein contains all or some identical subunits. Monomers and polymers are irrelevant in the quaternary structure.
13. Find the odd one out?
a) Hydrogen bond
b) Disulfide bond
c) Ionic bond
d) Peptide bond
View Answer
Explanation: Hydrogen bond, disulfide bond, an ionic bond are the interactions that make up the quaternary structure. The peptide bond is the basis of the primary structure of a protein. Hence, the peptide bond is the odd one out.
14. Find the odd one out?
a) Collagen
b) Insulin
c) Hemoglobin
d) A chain of insulin
View Answer
Explanation: Collagen, insulin, and hemoglobin are multi-subunit proteins. Hence, they all contain a quaternary structure. A chain of insulin contains only a single polypeptide chain. Thus, it does not contain a quaternary structure. Therefore, A chain of insulin is the odd one out.
15. Which of the following is used for the reduction of disulfide bonds?
a) Urea
b) Guanidine hydrochloride
c) Iodoacetate
d) Beta-mercaptoethanol/ dithiothreitol
View Answer
Explanation: Beta-mercaptoethanol/dithiothreitol is used forthe reduction of disulfide bonds. Urea and guanidine hydrochloride are denaturating agents, while Iodoacetate is used for acetylation of sulfhydryl groups.
Sanfoundry Global Education & Learning Series – Protein Engineering.
To practice all areas of Protein Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
- Check Protein Engineering Books
