This set of Protein Engineering Objective Questions & Answers focuses on “Applications – Affinity Purification – 2”.
1. Which of the following is an 8-residue minimal peptide sequence that exhibits intrinsic affinity towards streptavidin and can be fused to recombinant proteins in various combinations?
a) Strep-tag I
b) S-tag
c) Flag peptide
d) Strep-tag II
View Answer
Explanation: Strep-tag II is an 8-residue minimal peptide sequence that exhibits intrinsic affinity towards streptavidin and can be fused to recombinant proteins in various combinations. Strep-tag I cannot be fused to recombinant proteins in various combinations. Efficient procedures for purification, detection, and immobilization or separation are of key importance in modern protein science.
2. Strep-tag II hampers protein folding or secretion and it usually interferes with protein function.
a) True
b) False
View Answer
Explanation: The above statement is false. A particular benefit of the Strep-tag II is that it does not hamper protein folding orsecretion and it usually does not interfere with protein function. Thus, it is optimally suited for the preparation and analysis of functional proteins.
3. Which of the following is a homo-tetramer of 159 amino acids per subunit?
a) Biotin
b) Flag peptide
c) S-tag
d) Streptavidin
View Answer
Explanation: Streptavidin is a homo-tetramer of 159 amino acids per subunit. It possesses four independent biotin-binding sites with Kd values as low as 10-14. Biotin, flag peptide, or S-tag are not homo-tetramers of 159 amino acids per subunit.
4. Strep-tag II is sensitive to cellular proteases.
a) True
b) False
View Answer
Explanation: The above statement is false. Strep-tag II is resistant to cellular proteases. Hence, it can be used in the presence of mild detergents and it is biochemically almost inert. Strep-tag II is a synthetic peptide consisting of 8 amino acids.
5. Streptavidin is naturally secreted by which of the following microorganisms?
a) Aspergillus niger
b) Escherichia coli
c) Bacillus anthrax
d) Streptomyces avidinii
View Answer
Explanation: Streptavidin is naturally secreted by Streptomyces avidinii. It is secreted as a complex with a small antibiotic molecule. Aspergillus niger, Escherichia coli, and Bacillus anthrax naturally do not secrete streptavidin. Streptavidin is a homo-tetramer and has an extraordinary binding affinity for biotin.
6. An ideal affinity tag should interfere with the folding or bioactivity of a recombinant protein.
a) True
b) False
View Answer
Explanation: The above statement is false. An ideal affinity tag should not interfere with the folding or bioactivity of a recombinant protein. Moreover, an ideal affinity tag should allow a high purification yield. These properties make it well-suited for studying functional proteins.
7. Which of the following affinity tag is shown in the figure below?
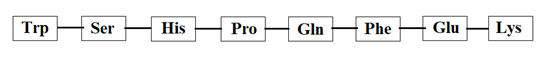
a) Strep-tag I
b) Flag peptide
c) Myc tag
d) Strep-tag II
View Answer
Explanation: The affinity tag shown in the above figure is Strep-tag II. Over the years, both the peptide (Strep-tag I and Strep-tag II) and streptavidin core (Strep-Tactin) have been engineered for optimal binding affinity. The peptide shown in the figure above is not of Strep-tag I, Flag peptide, or Myc tag.
8. The affinity of Strep-tag for streptavidin is very high when it is placed at the amino terminus or in between two protein domains.
a) True
b) False
View Answer
Explanation: The above statement is false. Although the strep-tag proved to be useful for the detection and purification of a variety of recombinant proteins when attached to their carboxy-terminal end, its affinity for streptavidin was substantially diminished when placed at the amino terminus or in between two protein domains.
9. Which of the following fits in the empty box in the figure below?
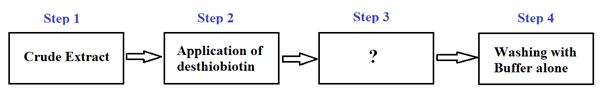
a) Application of NaOH
b) Application of Biotin
c) Application of Strep-Tactin
d) Application of HABA
View Answer
Explanation: “Application of HABA” fits in the empty box in the figure above. HABA (Yellow in colour) displaces the desthiobiotin and binds to the Strep-Tactin. Displacement of desthiobiotin is complete when the column has turned red. Application of NaOH, Application of Biotin, or Application of Strep-Tactin do not fit in the empty box above.
10. Which of the following is used in the column regeneration step of Strep-tag purification, that displaces desthiobiotin?
a) Buffer
b) Biotin
c) NaOH
d) HABA
View Answer
Explanation: Hydroxyazobenzoic acid (HABA) is used in the column regeneration step of Strep-tag purification, that displaces desthiobiotin. Application of HABA (yellow) displaces the desthiobiotin and turns the column red, due to the formation of hydrazone isomer. Buffer, Biotin, or NaOH are not used in the column regeneration step of Strep-tag purification, that displaces desthiobiotin.
11. Which of the following is an engineered streptavidin variant?
a) Biotin
b) Strep-tag II
c) Strep-tag I
d) Strep-Tactin
View Answer
Explanation: Strep-Tactin is an engineered streptavidin variant. It has a very high binding affinity to Strep-tag II. The Strep-tag system is one of the most widely used affinity chromatography systems for protein purification and detection and immobilization. Biotin, Strep-tag II, or Strep-tag I are not engineered streptavidin variant.
12. Which of the following shows the highest binding affinity for Strep-Tactin?
a) Biotin
b) Strep-tag I
c) Strep-tag II
d) Twin-strep-tag
View Answer
Explanation: Twin-strep-tag shows the highest binding affinity for Strep-Tactin. Although Biotin, Strep-tag I, and Strep-tag II show an affinity for Strep-Tactin, their binding affinity is weaker compared to Twin-strep-tag. Twin-strep-tag is a 28 amino acid peptide, containing two Strep-tag II motifs in series.
13. Which of the following is used in the column regeneration step of Twin-Strep-tag: Strep-Tactin XT purification, that displaces biotin?
a) Desthiobiotin
b) Buffer
c) HABA
d) NaOH
View Answer
Explanation: NaOH is used in the column regeneration step of Twin-Strep-tag : Strep-Tactin XT purification, that displaces biotin. It regenerates the column for the next cycle of protein purification. Desthiobiotin, Buffer, or HABA are not used in the column regeneration step of Twin-Strep-tag : Strep-Tactin XT purification.
14. Strep-tag I can be used at which of the following positions?
a) Carboxy or amino-terminal
b) Amino-terminal
c) In between two protein domains
d) Carboxy terminal
View Answer
Explanation: Strep-tag I can be used at carboxy-terminal of the protein. It cannot be used at amino-terminal or in between two protein domains. The affinity of Strep-tag I for streptavidin was substantially diminished when placed at the amino terminus or in between two protein domains.
15. An ideal affinity tag should display an easily controllable binding behavior with sufficient affinity.
a) False
b) True
View Answer
Explanation: The above statement is true. An ideal affinity tag should display an easily controllable binding behavior with sufficient affinity for streptavidin. Moreover, ideal affinity tags should not require harsh conditions for elution/washing.
Sanfoundry Global Education & Learning Series – Protein Engineering.
To practice all objective questions on Protein Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Biotechnology Books
- Check Protein Engineering Books
- Practice Biotechnology MCQs
- Apply for Biotechnology Internship
