This set of Protein Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Structure Function Relationships – Integral Membrane Proteins”.
1. Which of the following protein is also known as intrinsic proteins?
a) Peripheral proteins
b) Lipid-anchored proteins
c) Intracytoplasmic proteins
d) Integral membrane proteins
View Answer
Explanation: Integral membrane proteins are is also known as intrinsic proteins. These proteins are held in the bilayer by unusually tight binding and cannot be released by relatively gentle disruption procedures. Hence, these proteins are termed as integral membrane proteins or intrinsic proteins.
2. Which of the following is not a transmembrane protein?
a) Bacteriorhodopsin
b) GPCR
c) Glycophorin
d) G protein
View Answer
Explanation: G protein is not a transmembrane protein. It is a lipid anchored protein; hence it is loosely attached to the plasma membrane. It is always present on the inner leaflet on the plasma membrane. Bacteriorhodopsin, GPCR, and glycophorin are transmembrane proteins.
3. Which of the following type of protein is shown in the figure below?
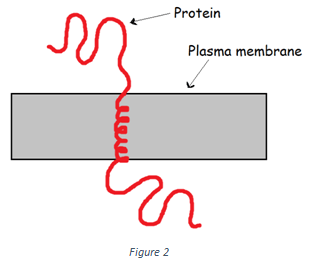
a) Polytopic transmembrane proteins
b) Lipid-anchored proteins
c) Peripheral proteins
d) Monotopic transmembrane proteins
View Answer
Explanation: The protein showed in the figure above belongs to the class monotopic transmembrane proteins. Monotopic transmembrane proteins contain only one transmembrane segment as depicted in the diagram. An example of monotopic transmembrane protein is Glycophorin.
4. Transmembrane proteins belong to which class of proteins?
a) Peripheral proteins
b) Lipid-anchored proteins
c) Extrinsic proteins
d) Integral membrane proteins
View Answer
Explanation: Transmembrane proteins belong to Integral membrane proteins. Most of the integral membrane proteins have a transmembrane segment. Many integral membrane proteins span the entire phospholipid bilayer.
5. Which of the following is an example of integral membrane proteins?
a) Spectrin
b) Actin
c) G proteins
d) GPCRs
View Answer
Explanation: GPCRs are examples of integral membrane proteins. GPCR stands for G protein-coupled receptor. These receptors are also known as serpentine receptors because they contain seven transmembrane helices that span the plasma membrane. Spectrin, actin, and G proteins are not examples of integral membrane proteins.
6. The hydrophobic helix is prevented from slipping across the membrane by flanking a set of which amino acids?
a) Serine and methionine
b) Glycine and valine
c) Alanine and tyrosine
d) Lysine and arginine
View Answer
Explanation: The hydrophobic helix is prevented from slipping across the membrane by flanking a set of lysine and arginine amino acids. Lysine and arginine are positively charged basic amino acids. Hence, they are thought to interact with negatively charged phospholipid head groups.
7. Which of the following type of protein is shown in the figure below?
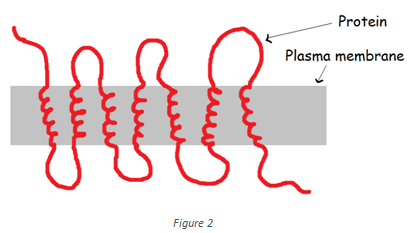
a) Peripheral proteins
b) Lipid-anchored proteins
c) Monotopic transmembrane proteins
d) Polytopic transmembrane proteins
View Answer
Explanation: The protein shown in the above figure belongs to the class of polytopic transmembrane proteins. As shown in the diagram these proteins pass through the plasma membrane more than once. Hence, they contain more than one transmembrane segment. Transmembrane proteins can be a single pass (monotopic) or multi-pass (polytopic).
8. Which of the following is a single-pass transmembrane protein?
a) Carbonate-bicarbonate exchanger
b) Glucose permease
c) GPCR
d) Glycophorin
View Answer
Explanation: Glycophorin is a single-pass transmembrane protein. It is a small protein as it contains only 131 amino acids. It is a glycoprotein. Glycophorin contains only one transmembrane segment. Carbonate-bicarbonate exchanger, glucose permease, and GPCR are multi-pass transmembrane proteins.
9. Which of the following proteins do not have alpha-helix in their transmembrane portion?
a) Rhodopsin
b) GPCRs
c) Glycophorins
d) Porins
View Answer
Explanation: Porins do not have alpha-helix in their transmembrane portion. They contain beta-barrels in their transmembrane portion. Beta-barrels are made up of anti-parallel beta-sheets. Rhodopsin, GPCRs, and glycophorins have alpha-helix in their transmembrane portion.
10. Which of the following type of proteins are not released from the membrane by relatively gentle extraction procedures, such as exposure to solutions of very high or low ionic strength or of extreme pH?
a) Peripheral proteins
b) Lipid-anchored proteins
c) Extrinsic proteins
d) Intrinsic proteins
View Answer
Explanation: Intrinsic proteins are not released from the membrane by relatively gentle extraction procedures, such as exposure to solutions of very high or low ionic strength or extreme pH. The exposures interfere with protein-protein interactions but leave the lipid bilayer intact.
11. The alpha-helices of transmembrane proteins are composed entirely of hydrophobic amino acid residues.
a) False
b) True
View Answer
Explanation: The above statement is true. The alpha-helices of transmembrane proteins are composed entirely of hydrophobic amino acid residues. The hydrophobic side chains form van der Waals interactions with the fatty acyl chains and shield the polar carbonyl and imino groups of the peptide bond.
12. The interior of beta-barrels in porins is hydrophilic.
a) False
b) True
View Answer
Explanation: The above statement is true. The interior of beta-barrels in porins is hydrophilic. Porins contain beta-barrel in their transmembrane segment. Beta-barrels are made up of anti-parallel beta-strands. The beta-barrel is stabilized by extensive hydrogen bonding between the beta-strands. The interior of the barrel is hydrophilic.
13. Which was the first transmembrane protein for which the complete amino acid sequence was determined?
a) ATPase
b) GPCR
c) Rhodopsin
d) Glycophorin
View Answer
Explanation: Glycophorin was the first transmembrane protein for which the complete amino acid sequence was determined. It is a major single-pass transmembrane protein of RBC. Glycophorin is a small protein as it contains only 131 amino acid residues.
14. Which of the following is not a multi-pass transmembrane protein?
a) Glucose permease
b) GPCR
c) Rhodopsin
d) Glycophorin
View Answer
Explanation: Glycophorin is not a multi-pass transmembrane protein. Glycophorin is a small single-pass transmembrane protein and it consists of only 131 amino acid residues. Glucose permease, GPCR, and rhodopsin are multi-pass transmembrane proteins.
15. Extrinsic proteins are held tightly to the lipid bilayer.
a) True
b) False
View Answer
Explanation: The above statement is false. Extrinsic proteins are not held tightly to the lipid bilayer. Whereas they are held loosely to the lipid bilayer. Intrinsic proteins are held tightly to the plasma membrane.
Sanfoundry Global Education & Learning Series – Protein Engineering.
To practice all areas of Protein Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Protein Engineering Books
- Check Biotechnology Books
- Apply for Biotechnology Internship
- Practice Biotechnology MCQs
