This set of Basic to Chemical Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Recycle without Chemical Reaction”.
1. How many recycle streams are there in the following process?

a) 1
b) 2
c) 3
d) 4
View answer
Explanation: 2 recycle streams are there which goes from second reactor to first.
2. How many streams are there in the following process?
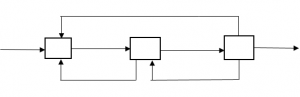
a) 1
b) 2
c) 3
d) 4
View answer
Explanation: There are three recycle streams, from second to first, from third to first, from second to first.
3. How many recycle streams are there in the following process?
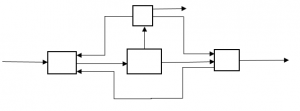
a) 1
b) 2
c) 3
d) 4
View answer
Explanation: There are two recycle streams, one from top reactor to left most reactor and other from right most to left most.
4. What is the value of P, if the value of A = 10 mole, B = 15 mole, C = 6 mole, D = 8 mole?

a) 2 mole
b) 5 mole
c) 6 mole
d) 8 mole
View answer
Explanation: Material balance for whole process, A = P + D, => P = 2 mole.
5. In the diagram below, what is the left most box?
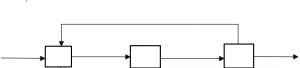
a) Mixer
b) Separator
c) Processor
d) None of the mentioned
View answer
Explanation: Any reactor in which recycled matter goes in is a mixer.
Answer question 6 – 10 for the following diagram.
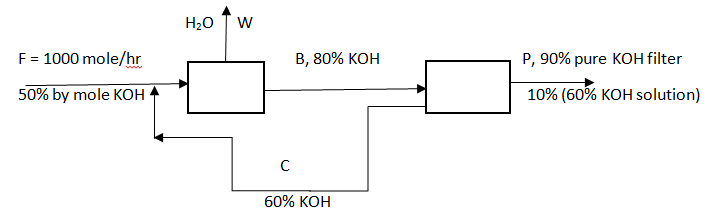
6. What is the value of P?
a) 105.4 mole/hr
b) 254.3 mole/hr
c) 412.1 mole/hr
d) 520.8 mole/hr
View answer
Explanation: Overall NaOH balance, 1000*0.5 = P [0.9*1 + 0.1*0.6], => P = 520.8 mole/hr.
7. What is the value of W?
a) 227.5 mole/hr
b) 479.1 mole/hr
c) 568.2 mole/hr
d) 721.4 mole/hr
View answer
Explanation: Overall water balance, 1000*0.5 = 520.8*0.1*0.6 + W*1, => W = 479.1 mole/hr.
8. What is the value of C?
a) 104.1 mole/hr
b) 213.5 mole/hr
c) 342.2 mole/hr
d) 416.4 mole/hr
View answer
Explanation: First reactor balance, KOH: 1000*0.5 + C*0.6 = B*0.8, => 4B = 3C + 2500, H2O: 1000*0.5 + C*0.4 = 479.1*1 + B*0.2, => 209 + 4C = 2B, solving both equations we get C = 416.4 mole/hr.
9. What is the value of C?
a) 945.5 mole/hr
b) 1874.6 mole/hr
c) 2474.5 mole/hr
d) 3951.2 mole/hr
View answer
Explanation: First reactor balance, KOH: 1000*0.5 + C*0.6 = B*0.8, => 4B = 3C + 2500, => B = 1874.6 mole/hr.
10. What is the value of F for the B solution to remain 80% KOH if the recycle stream is removed?
a) 123.1 mole/hr
b) 666.5 mole/hr
c) 988.4 mole/hr
d) 1499.8 mole/hr
View answer
Explanation: Second reactor balance, KOH: B*0.8 = C*0.6 + 520.8*[0.9*1 + 0.1*0.6], => 4B = 3C + 2499.8, H2O: B*0.2 = C*0.4 + 520.8*[0.1*0.4], => B = 2C + 104.1, solving both equations we get B = 937.4 mole/hr. First reactor balance, KOH: F*0.5 = 937.4*0.8, => F = 1499.8 mole/hr.
Answer question 11 – 15 for the following diagram.

11. What is the value of P?
a) 14.4 mole/hr
b) 36.3 mole/hr
c) 48.1 mole/hr
d) 65.9 mole/hr
View answer
Explanation: Overall NaCl balance: 100*0.05 = B*0.12 + P*0.01, => 12B + P = 500, Overall water balance: 100*0.95 = B*0.88 + P*0.99, => 88B + 99P = 9500, => Solving both equations we get P = 36.3 mole/hr.
12. What is the value of B?
a) 18.9 mole/hr
b) 38.6 mole/hr
c) 45.2 mole/hr
d) 72.1 mole/hr
View answer
Explanation: Overall NaCl balance: 100*0.05 = B*0.12 + P*0.01, => 12B + P = 500, => B = 38.6 mole/hr.
13. What is the value of H?
a) 250 mole/hr
b) 400 mole/hr
c) 525 mole/hr
d) 750 mole/hr
View answer
Explanation: First reactor balance, NaCl: 100*0.05 + G*0.1 = H*0.08, => 8H = 10G + 500, H2O: 100*0.95 + G*0.9 = H*0.92, => 92H = 90G + 9500, Solving both equations we get H = 250 mole/hr.
14. What is the value of G?
a) 50 mole/hr
b) 100 mole/hr
c) 150 mole/hr
d) 200 mole/hr
View answer
Explanation: First reactor balance, NaCl: 100*0.05 + G*0.1 = H*0.08, => 8H = 10G + 500, => G = 150 mole/hr.
15. What is the value of C?
a) 126.1 mole/hr
b) 150.8 mole/hr
c) 178.4 mole/hr
d) 196.3 mole/hr
View answer
Explanation: Processor balance, NaCl: 0.08*250 = 0.01*36.3 + 0.1*C, => C = 196.3 mole/hr.
Sanfoundry Global Education & Learning Series – Basic Chemical Engineering.
To practice all areas of Basic Chemical Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
