This set of Basic Chemical Engineering Multiple Choice Questions & Answers (MCQs) focuses on “Bypass and Purge”.
1. A bypass stream does not go through which of the following?
a) Mixer
b) Process
c) Separator
d) None of the mentioned
View answer
Explanation: A bypass stream directly goes from divider to separator skipping the process.
2. Which of the following does the purge stream comes from?
a) Feed stream
b) Product stream
c) Recycle stream
d) None of the mentioned
View answer
Explanation: Purge stream is the removal of unwanted material from the recycle stream.
3. Which of the following composition can be controlled by a bypass stream?
a) Exit stream
b) Feed
c) Process
d) None of the mentioned
View answer
Explanation: Bypass stream can be used to control the composition of a final stream.
4. Which of the following composition is controlled by purge stream?
a) Feed
b) Product stream
c) Recycle stream
d) None of the mentioned
View answer
Explanation: Composition of recycle stream can be controlled by purge stream.
5. Which of the following is decreased by the bypass stream?
a) Feed
b) Product
c) Feed & Product
d) None of the mentioned
View answer
Explanation: Bypass stream decreases the feed and increases the product.
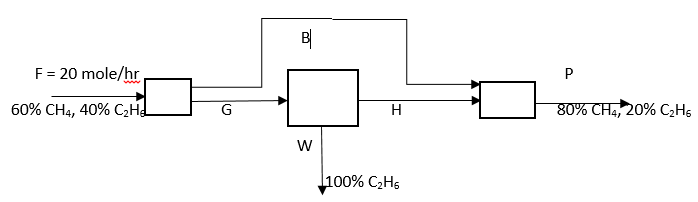
Answer question 6 – 10 for the following diagram.
6. What is the value of P?
a) 5 mole/hr
b) 10 mole/hr
c) 15 mole/hr
d) 20 mole/hr
View answer
Explanation: Overall CH4 balance: 0.6*20 = 0.8*P, => P = 15 mole/hr.
7. What is the value of W?
a) 5 mole/hr
b) 10 mole/hr
c) 20 mole/hr
d) 25 mole/hr
View answer
Explanation: Overall C2H6 balance: 0.4*20 = 0.2*15 + 1*W, => W = 5 mole/hr.
8. What is the value of G?
a) 12.5 mole/hr
b) 25 mole/hr
c) 37.5 mole/hr
d) 50 mole/hr
View answer
Explanation: Material balance: G = 5 + H, CH4 balance: G(0.6) = H, solving both equations G = 12.5 mole/hr.
9. What is the value of H?
a) 5 mole/hr
b) 7.5 mole/hr
c) 10 mole/hr
d) 12.5 mole/hr
View answer
Explanation: H = 0.6G = 7.5 mole/hr.
10. What is the value of B?
a) 7.5 mole/hr
b) 15 mole/hr
c) 20 mole/hr
d) 22.5 mole/hr
View answer
Explanation: B = F – G = 20 – 12.5 = 7.5 mole/hr.
Answer question 11 – 15 for the following diagram. The reaction is N2 + 3H2 -> 2NH3, overall conversion of N2 is 80%.
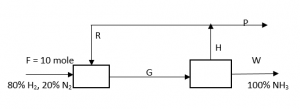
11. What is the value of W?
a) 2.5 mole
b) 5 mole
c) 9.5 mole
d) 20 mole
View answer
Explanation: 0.2*10 = 0.8*1*W, => W = 2.5 mole.
12. What is the value of P?
a) 2.5 mole
b) 5 mole
c) 7.5 mole
d) 10 mole
View answer
Explanation: F = W + P, => 10 = 2.5 + P, => P = 7.5 mole.
13. What is the fraction of N2 in purge stream?
a) 0.04
b) 0.05
c) 0.16
d) 0.4
View answer
Explanation: N2 balance: 10*0.2 – 10*0.2*0.8 = 7.5*x, => x = 0.05.
14. How many moles of H2 are recycled?
a) 1.5
b) 2.2
c) 2.8
d) 3.9
View answer
Explanation: Moles of H2 in G stream = 10*0.8 – 3*10*0.2*0.8 = 3.2 = moles of H2 in H stream. Moles of H2 in P stream = 0.95*7.5 = 7.125, => moles of H2 in R stream = 7.125 – 3.2 = 3.925.
15. What is the value of R?
a) 1.4
b) 2.8
c) 4.2
d) 6.8
View answer
Explanation: Recycled moles of H2 = 3.925, recycled moles of N2 = (10*0.2 – 10*0.2*0.8) – 0.05 = 0.35, => R = 3.925 + 0.35 = 4.275.
Sanfoundry Global Education & Learning Series – Basic Chemical Engineering.
To practice all areas of Basic Chemical Engineering, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]