This set of Refrigeration Quiz focuses on “Theoretical VCR with Dry Saturated Vapour after Compression – 2”.
1. Refrigerating effect corresponds to which of the following processes?
a) Condensing process
b) Vaporizing process
c) Compression process
d) Expansion process
View Answer
Explanation: During evaporation, the refrigerant absorbs its latent heat of vaporization from the medium or system which is to be cooled. So, the heat absorbed by the refrigerant is known as Refrigerating effect carried out in the vaporizing process.
2. What does the process 1-2 represent?
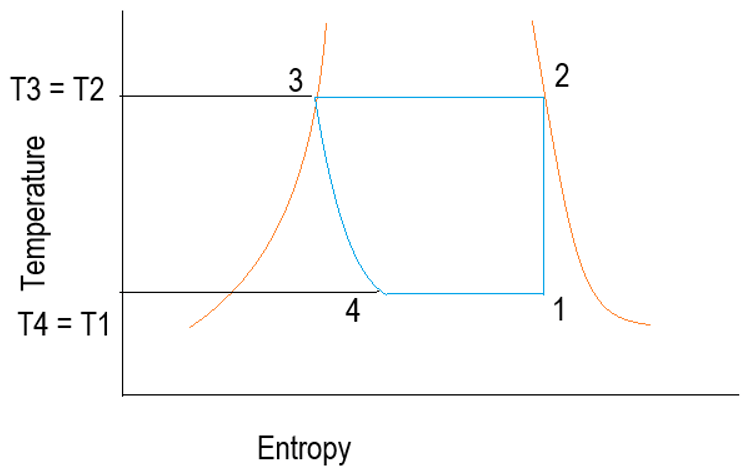
a) Condensation
b) Evaporation
c) Compression
d) Expansion
View Answer
Explanation: As from 1-2 temperature is increasing by keeping change in entropy constant. By Gay-Lussac’s law, the pressure is proportional to temperature. Hence, pressure also increasing from point 1-2. It represents the compression process where pressure is increased.
3. What does the process 2-3 represent?

a) Condensation
b) Evaporation
c) Compression
d) Expansion
View Answer
Explanation: As in the process 2-3, the temperature is constant by decreasing the change in entropy. As entropy change is related to heat transfer the second law of thermodynamics. Change in entropy is reduced. Hence, heat is rejected. So, it is a condensation process.
4. What does the process 3-4 represent?

a) Condensation
b) Evaporation
c) Compression
d) Expansion
View Answer
Explanation: As in the process 3-4, the temperature is decreased and also decrease in an optimal change in entropy. As pressure is reduced as well as optimal heat transfer is carried out to get the desired phase. These both processes are observed in Expansion, not only decreasing pressure is essential but also stabilizing the randomness of particles.
5. What does the process 2-3 represent?

a) Condensation
b) Evaporation
c) Compression
d) Expansion
View Answer
Explanation: As in the process 4-1, the temperature is constant by increasing the change in entropy. As entropy change is related to heat transfer the second law of thermodynamics. Change in entropy is increased by increasing the randomness done by phase change. Hence, heat is absorbed. So, it is an evaporation process.
6. What does point 2 say about the state of refrigerant?

a) Wet vapor
b) Dry saturated vapor
c) Superheated vapor
d) Liquid refrigerant
View Answer
Explanation: Point 2 lies on the saturated vapor line. Hence, it is a dry saturated vapor containing no liquid particles. If the point is inside the curve, then it is wet vapor. If it is outside the line, it is superheated vapor. It cannot be liquid after compression.
7. What is the value of C.O.P. for the following p-h chart?
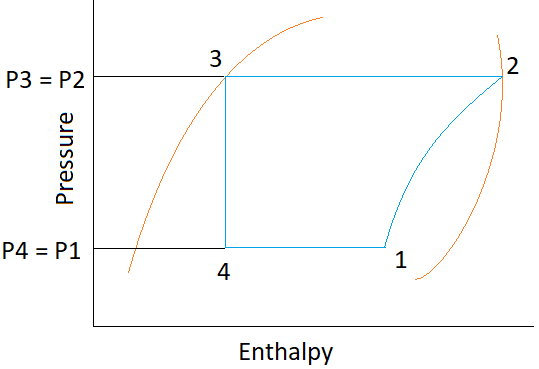
a) (h1 − h4) / (h2 − h1)
b) (h3 − h4) / (h2 − h1)
c) (h2 − h4) / (h2 − h3)
d) (h1 − h2) / (h3 − h4)
View Answer
Explanation: C.O.P. = Refrigerating effect / Work done
As, Refrigerating effect = Process 1-4 = h1 − h4
Work done = Process 1-2 = h2 − h1
Hence, C.O.P. = (h1 − h4) / (h2 − h1).
8. Find out the value of C.O.P. for the given values of enthalpies.
h1 = 1126.25 kJ/kg
h4 = 250.25 kJ/kg
W = 120 kJ/kg
a) 5.3
b) 6.3
c) 7.3
d) 8.3
View Answer
Explanation: Refrigerating effect = h1 − h4 = 1126.25 − 250.25 = 876 kJ/kg
Work = 120 kJ/kg
C.O.P. = Refrigerating effect / Work done
= 876 / 120
= 7.3.
9. If a process 1 – 2 undergoes a throttling. Which of the following represents the process 1 – 2?
a) T1 = T2
b) S1 = S2
c) P1 = P2
d) H1 = H2
View Answer
Explanation: In a throttling process, no heat is absorbed or rejected by the liquid refrigerant. It is also called as an isenthalpic process, in which change in internal energy and flow work remains the same. Enthalpies remain same, so H1 = H2 represents throttling.
T1 = T2 is the isothermal process.
S1 = S2 is an isentropic process.
P1 = P2 is an isobaric process.
10. In a vapor compression refrigeration system, the coefficient of performance is observed as 10. If the enthalpy after compression is 1368.28 kJ/kg and after condensation is 598.58 kJ/kg. If the system works on 50% of the C.O.P., then what is the value of enthalpy after evaporation?
a) 1220
b) 1210
c) 1240
d) 1280
View Answer
Explanation: Given data: Theoretical C.O.P. = 10
h2 = 1368.28 kJ/kg
h4 = h3 = 598.58 kJ/kg
Calculations: Actual C.O.P. = 50 * 10 / 100 = 5
As, C.O.P. = (h1 − h4) / (h2 − h1)
5 = (h1 − 598.58) / (1368.28 − h1)
h1 = 1239.99 = 1240 kJ/kg.
Sanfoundry Global Education & Learning Series – Refrigeration & Air Conditioning.
To practice all areas of Refrigeration for Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mechanical Engineering Books
- Check Refrigeration & Air Conditioning Books
- Practice Mechanical Engineering MCQs
- Apply for Mechanical Engineering Internship
