This set of Mass Transfer Multiple Choice Questions & Answers focuses on “Distillation-Differential Distillation (Rayleigh Equation)”.
1. Large number of flash distillation equals a differential distillation.
a) True
b) False
View Answer
Explanation: In flash vaporization, only a small portion of liquid gets flashed.
2. The column used for differential distillation is_______________
a) Still
b) Differential column
c) Batch column
d) None of the mentioned
View Answer
Explanation: Still is the distillation column used for differential distillation.
3. Simple distillation is a___________ process.
a) Batch
b) Continuous
c) Adiabatic
d) None of the mentioned
View Answer
Explanation: Here, the liquid mixture is filled in the still for a certain time to attain the vapour liquid equilibrium.
4. The initial portion of the distillate will be a low volatile component.
a) True
b) False
View Answer
Explanation: Since the low volatile component has a high boiling point it will be the final portion.
5. After the separation by differential distillation, the high volatile and low volatile components are stored in _____________
a) Batch
b) Cuts
c) Distill
d) None of the mentioned
View Answer
Explanation: Cuts are the place where the different volatile components are stored.
6. For a ternary mixture, the number of cuts required is_________
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: The first two high volatile components are stored in two cuts and the low volatile remains in the still itself.
7. The differential distillation in order to maintain the equilibrium the process should be slow.
a) True
b) False
View Answer
Explanation: For the differential distillation to get the effective distillation the process must be slow to attain the vapour- liquid equilibrium.
8. Pre-cooling is required before passing the vapour to the condenser in a still.
a) True
b) False
View Answer
Explanation: If pre-cooling occurs, the vapour gets condense in the still itself so there will be alter in equilibrium.
9. The equation applicable for batch distillation is _________
a) Frenske’s equation
b) Rayleigh equation
c) Wilke-chan equation
d) None of the mentioned
View Answer
Explanation: Rayleigh proposed the numerical analysis of the batch distillation.
10. Estimate the feed rate, if the composition of the more volatile component in the feed is 50% and the residue is 10% and the equilibrium relation is given by y*= 1.975x. Also, the rate of residue is 60 mol/hr.
a) 213 mol/hr
b) 313 mol/hr
c) 413 mol/hr
d) 513 mol/hr
View Answer
Explanation: Using Rayleigh equation, we can estimate
ln (F/W)= 1.657
F= 313 mol/hr.
11. Find the feed rate by analysing the graph,
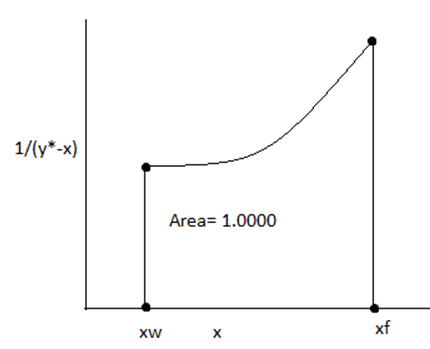
a) 153 mol/hr
b) 163 mol/hr
c) 173 mol/hr
d) 183 mol/hr
View Answer
Explanation: Using Rayleigh equation,
Ln (F/W) = Area under the curve
= 1
F= 163.
12. Find the composited distillate composition,
| Mol/hr | ||
|---|---|---|
| Feed | 100 | 0.45 |
| Distillate | 60 | 0.95 |
| Residue | 40 | 0.2 |
a) 0.52
b) 0.62
c) 0.72
d) 0.82
View Answer
Explanation: F*xf= D*yd+ W*xw
Yd= 0.62.
13. Find the value of relative volatility for a constant pressure process if
| Rate(mol/s) | Composition | |
|---|---|---|
| Feed | 100 | 45% |
| Residue | 30 | 20% |
a) 1
b) 2
c) 2.43
d) Data inadequate
View Answer
Explanation: For a constant pressure process, the will be a constant relative volatility. So from modified Rayleigh method we can determine relative volatility,
Log(F*xf/W*xw)= A log ( F(1-xf)/W(1-xw)), where A- constant relative volatility
A= 2.43.
14. Find the distillate rate, if a differential condenser is used and the equilibrium relation is given as x*= 0.2y. Also the rate of feed is 100 mol/hr.
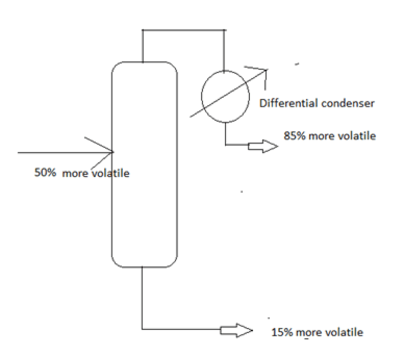
a) 12 mol/hr
b) 22 mol/hr
c) 33 mol/hr
d) 44 mol/hr
View Answer
Explanation: From the Rayleigh equation for differential condenser, we get distillate rate = 22.
15. Find the rate of distillate, if the composition of the more volatile component in the feed is 50% and the residue is 10% and the equilibrium relation is given by y*= 1.975x. Also the rate of residue is 60 mol/hr.
a) 153 mol/hr
b) 253 mol/hr
c) 353 mol/hr
d) 453 mol/hr
View Answer
Explanation: Using the Rayleigh equation, we can estimate
ln (F/W)= 1.657
F= 313 mol/hr then
D= 253 mol/hr.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice multiple choice questions in all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mechanical Engineering Books
- Practice Chemical Engineering MCQs
- Check Mass Transfer Books
- Practice Mechanical Engineering MCQs
- Apply for Chemical Engineering Internship
