This set of Mass Transfer quiz focuses on “Distillation-Vapour Liquid Equilibria”.
1. A new phase is given for the separation by distillation.
a) True
b) False
View Answer
Explanation: Here, the separation is done by vaporization.
2. Consider a sugar solution[ Sugar + Water]; On vaporization water only evaporate since sugar is________
a) Volatile
b) Non-Volatile
c) Cryogenic
d) None of the mentioned
View Answer
Explanation: There is no boiling point for the Non-volatile compound. So the sugar has no vapour pressure.
3. Distillation is possible only if the solution components are_____________
a) Volatile
b) Non-volatile
c) Cryogenic
d) None of the mentioned
View Answer
Explanation: Since the distillation is done by vaporization the components should be volatile for separation.
4. No further process required for _____________
a) Absorption
b) Extraction
c) Distillation
d) All of the mentioned
View Answer
Explanation: Distillation is the only process gives the pure component separation.
5. Look out the distribution curve, if the line PM is lesser than shown the separation is more
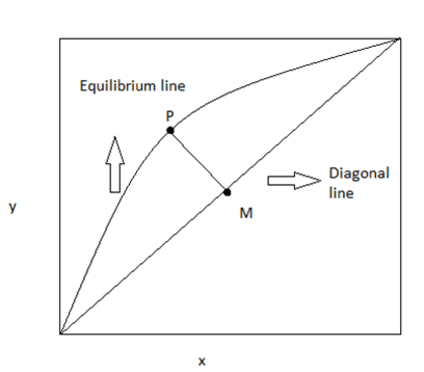
Where, x,y are the concentrations in mole fractions
a) True
b) False
View Answer
Explanation: The separation will be less. Since the distribution curve represents the volatility variance.
6. In a solution (A and B); The vapour pressure of A is 260 mmHg and B is 360 mmHg; Find the relative volatility?
a) 0.72
b) 1.38
c) 2
d) None of the mentioned
View Answer
Explanation: Relative volatility= vapour pressure of A/ Vapour pressure of B
= 260/360
=0.72.
7. If the gas phase composition of a component A is 0.65 and its relative volatility is 2. Find the liquid phase composition.
a) 0.48
b) 0.58
c) 0.68
d) 0.78
View Answer
Explanation: Relative volatility= 0.65/(1-0.65) * (1-x)/x
2= 1.85 * (1-x)/x ; x= 0.48.
8. The component A and B has the same boiling point. Can the separation is done by ordinary separation?
a) True
b) False
View Answer
Explanation: Separation cannot be done if the two components have the same vapour pressure.
9. Find the relative volatility, if x, y are the concentrations in mole fraction.
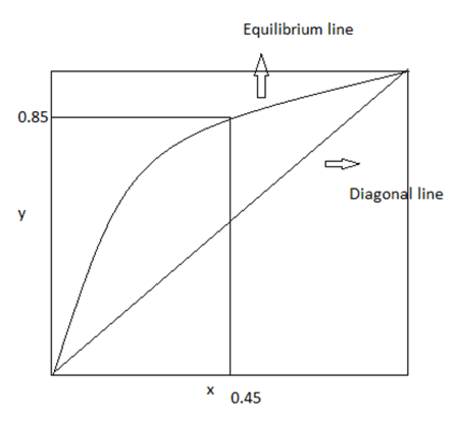
a) 6.92
b) 0.14
c) 1.88
d) None of the mentioned
View Answer
Explanation: y=0.85; x=0.45 ; relative volatility= (0.85/0.15) * (0.55/0.45) = 6.92.
10. The possibility of separation will be more if the separation factor is greater than 1.
a) True
b) False
View Answer
Explanation: Since if relative volatility is equals to 1. The separation is not possible since the boiling point of the two components will be same.
11. Find the vapour pressure of component A in an ideal mixture, if its liquid composition and the partial pressure is 0.65 and 260 mmHg.
a) 260 mmHg
b) 400 mmHg
c) 760 mmHg
d) Cannot determine
View Answer
Explanation: For an ideal solution, Partial pressure = vapour pressure x mole fraction
Vapour pressure= (260/0.65)=400 mmHg.
12. Consider an ideal vapour-liquid binary mixture, find the total pressure if the vapour pressure of A is 260 mmHg and the vapour pressure of B is 520 mmHg and the composition of A in mole fraction is 0.55.
a) 177 mmHg
b) 277 mmHg
c) 377 mmHg
d) 477 mmHg
View Answer
Explanation: Total pressure = (vapour pressure of A * mole fraction) + vapour pressure of B*( 1- mole fraction) = 377 mmHg.
13. Guess the exact relation between the vapour phase mole fraction and relative volatility.
a) y= A*x/(1+x(A+1))
b) y= A*x/(1-x(A-1))
c) y= A*x/(1+x(A-1))
d) y= A*x/(1-x(A+1))
View Answer
Explanation: A= (y/1-y)*((1-x)/x)
On rearranging, y= A*x/(1+x(A-1).
where, A – relative volatility.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer for quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Mechanical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Mechanical Engineering Internship
