This set of Mass Transfer Multiple Choice Questions & Answers (MCQs) focuses on “Flash Vaporization Line (q-line)”.
1. At what feed condition the slope of q line is infinity?
a) Saturated vapour
b) Saturated liquid
c) Superheated vapour
d) None of the mentioned
View Answer
Explanation: At saturated liquid the enthalpy of feed and the liquid is same so q=1 and slope = infinity.
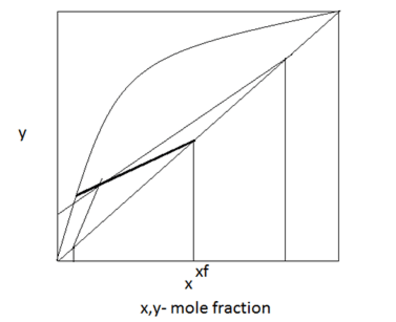
2. Find the feed condition from the below distribution curve.
a) Saturated vapour
b) Saturated liquid
c) Superheated vapour
d) None of the mentioned
View Answer
Explanation: Since the slope of the q-line is between 1 and 0 it is a superheated vapour.
3. In an operation the slope of the q-line is found to be 0; then the enthalpy of feed equals enthalpy of _____________
a) Liquid
b) Vapour
c) Mixture
d) None of the mentioned
View Answer
Explanation: Slope of q line = q/q-1
If the enthalpy of feed and vapour are equal then the q becomes zero; q line also becomes zero.
4. Consider a saturated liquid-vapour mixture, if the liquid flow rate inside the enriching and the stripping section of a fractionator is 50 and 40 mol/hr. Also the feed rate is 10 mol/hr. Find the feed condition.
a) Saturated vapour
b) Saturated liquid
c) Superheated vapour
d) None of the mentioned
View Answer
Explanation: q= 50-40/10 = 1; then the feed condition is a saturated liquid.
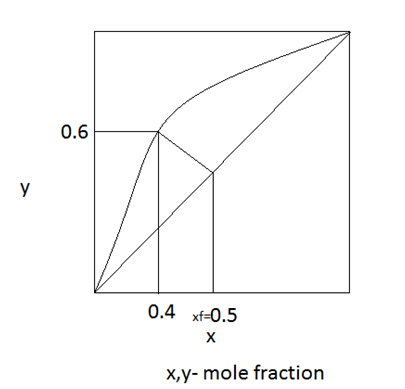
5. Find the liquid vapour compositions.
a) 0.5-Liquid & 0.5-Vapour
b) 0.6-Liquid & 0.4-Vapour
c) 0.4-Liquid & 0.6-Vapour
d) 0.5-Liquid & 0.4-Vapour
View Answer
Explanation: From the feed condition we can estimate that the feed has a mixture of liquid and vapour. From the distribution diagram, the vapour composition is 60% and the liquid composition is 40%.
6. At what feed condition the slope of the flash vaporization line becomes 0?
a) Saturated vapour
b) Saturated liquid
c) Superheated vapour
d) None of the mentioned
View Answer
Explanation: For a saturated vapour, the enthalpy of feed and the enthalpy of vapour is same so the value of q= 0; slope of q-line is 0.
7. The point where the operating line and the equilibrium line meets in distillation methods is known as
a) Plait point
b) Q point
c) Pinch point
d) None of the mentioned
View Answer
Explanation: At the pinch point, separation is not possible since it requires an infinite number of trays in the enriching section if operating and equilibrium line meets.
8. Find the exact q line for the saturated liquid from the distribution diagram,
a) op
b) oq
c) or
d) pr
View Answer
Explanation: For the saturated liquid, slope of q-line should be infinity.
9. The equilibrium composition of liquid and vapour in a feed can be identified by,
a) intersection of q line with equilibrium line
b) intersection of q line with operating line
c) intersection of operating line with equilibrium line
d) None of the mentioned
View Answer
Explanation: If the value of q is between 0 and 1 then the feed has both vapour and liquid. Then the compositions can be identified by intersecting q-line with operating line.
10. If the feed enters the distillation column at its dew point, then the slope of the Flash vaporization operating line is
a) 0
b) 1
c) Between 0 and 1
d) Infinity
View Answer
Explanation: If the feed at dew point means the feed condition is saturated vapour so the enthalpy of feed is same as the enthalpy of the vapour. Therefore the slope of the Q-line is 0. Since Q-line is the Flash vaporization operating line.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Mass Transfer Books
- Apply for Mechanical Engineering Internship
