This set of Mass Transfer Multiple Choice Questions & Answers (MCQs) focuses on “Cascade Stages”.
1. A device where two insoluble phases are brought into contact is known as
a) Trays
b) Stage
c) Cascade
d) Sieves
View Answer
Explanation: The stage is a single device where the phases are allowed to contact each other.
2. Ideal stages are also known as
a) Equilibrium or Theoretical stages
b) Equilibrium or Actual stages
c) Differential or Theoretical stages
d) Differential or Actual stages
View Answer
Explanation: Ideal stages are always equilibrium stages. Actual stages are practical stages hence theoretical stages are Ideal stages.
3. More than one stages are interconnected to form
a) Trays
b) Sieves
c) Cascades
d) Multiple stage
View Answer
Explanation: More than one stage is cascades.
4. The main purpose of cascades is
a) To get maximum conversion
b) To increase mass transfer rates
c) To get more efficiency
d) None of the mentioned
View Answer
Explanation: Though all the above mention is applicable, the main purpose is to increase the extent of mass transfer.
5. (Yn- Yn-1)/ (Yn*-Yn-1) is
a) Fractional overall
b) Murphree stage
c) Stage
d) Tray
View Answer
Explanation: The leaving stream is in equilibrium with its actual concentration. It is known as Murphree stage efficiency.
6. Find the number of stage(s)
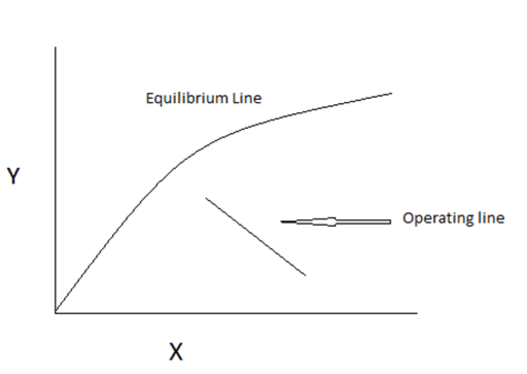
Where,
Y- Mole Ratio of gas phase
X- Mole Ratio of liquid phase
a) 1
b) 2
c) 1 to 2
d) No stage
View Answer
Explanation: one operating line = one stage.
7. Murphree efficiency is defined as the number of equilibrium stages to the number of real stages.
a) True
b) False
View Answer
Explanation: Fractional overall efficiency gives the ratio of equilibrium stage to real stage.
8. Find the value of y in an ideal single stage absorber is used to remove ammonia from gas with water as solvent. Its representation is given below
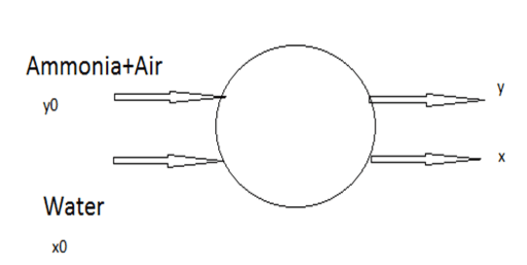
Here,
y0=0.2, the molar gas flow rates of liquid and gas mixture in (moles/sec) are 4 and 2. The equilibrium relation between y and x is given by y = 0.2x. The solvent water is pure.
a) 1/10
b) 1/20
c) 2/110
d) 2/220
View Answer
Explanation:
2 (0.2) + 4(0) = 2 (y*) + 4(x)
As y= 0.2x
0.4= 0.4x + 4x
x= (1/11)
y= 2/110.
9. ______________ is defined as the number of equilibrium stages to the number of real stages.
a) Murphree stage efficiency
b) Fractional overall efficiency
c) Stage efficiency
d) Overall efficiency
View Answer
Explanation: Fractional overall efficiency gives the ratio of equilibrium stage to real stage.
10. Find the Murphree stage efficiency for the following co-current operation with equilibrium leaving gas phase concentration Y*= 0.8.
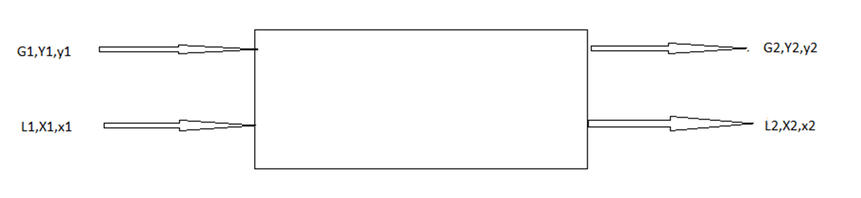
Where,
G and L are gas and liquid flow rates
Y and X are concentration in mole ratio (Y1=0.2, Y2=0.4, X1= 0.6, X2=0.8)
a) 25%
b) 50%
c) 75%
d) 100%
View Answer
Explanation: Murphree stage efficiency is= (Y2- Y1)/(Y2*- Y1)
Given gas phase leaving stream in equilibrium Y2*= 0.8
Then, Murphree stage efficiency is (0.2/0.8) x 100
Murphree stage efficiency is 25%.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mechanical Engineering Books
- Check Chemical Engineering Books
- Check Mass Transfer Books
- Apply for Mechanical Engineering Internship
- Practice Mechanical Engineering MCQs
