This set of Mass Transfer Multiple Choice Questions & Answers (MCQs) focuses on “Terinary Equilibrium Diagram”.
1. The extraction compositions in equilibrium are represented by equilateral triangle whose coordinates are known as
a) Isobar
b) Isotrope
c) Isotherm
d) None of the mentioned
View Answer
Explanation: The coordinates of the equilateral triangle are constant temperatures so it is an isotherm.
2. Find LPM in the below one pair partially miscible ternary diagram.
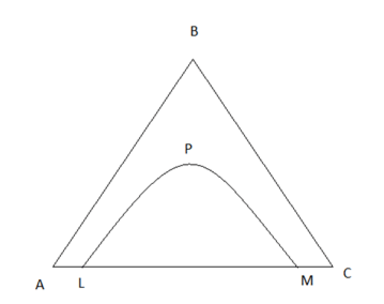
Where, A,B,C are the pure components
a) Temperature curve
b) Solubility Curve
c) Solution curve
d) Pressure curve
View Answer
Explanation: Solubility curve represent the components solubility on other component.
3. The change of solubility by adding other component gives rise to binodal solubility curve.
a) True
b) False
View Answer
Explanation: Binodal curve is the solubility curve of the ternary diagram.
4. Justify that X represents heterogeneity of the solution.
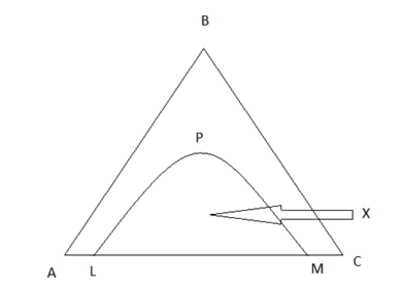
a) True
b) False
View Answer
Explanation: Here LPM represents the binodal solubility curve which represents the three component mixture.
5. The system becomes solutropic if the tie line inside the binodal solubility curves becomes_________
a) Vertical
b) Horizontal
c) Straight
d) None of the mentioned
View Answer
Explanation: If the tie line becomes horizontal the mixture solubility on each other remains same which represents solutropic system.
6. The point where A and B rich solubility curve merge is known as _________
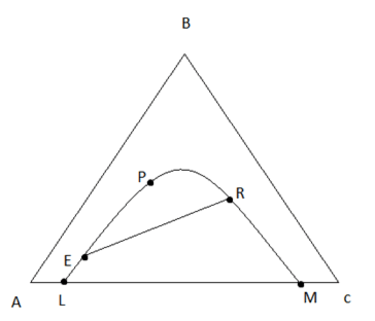
a) Pinch point
b) Plait point
c) Key point
d) None of the mentioned
View Answer
Explanation: Upon addition of B, the solubility of A and B rich phases meets is known as plait point where the separation is not possible.
7. Consider a solutropic system; the plait point will be at the maximum addition of? Find from the figure.
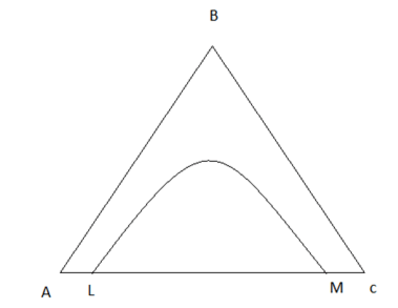
a) A
b) B
c) C
d) M
View Answer
Explanation: Upon addition of B the tie line will be horizontal for solutropic system and will be maximum at B.
8. Separation is not possible at plait point.
a) True
b) False
View Answer
Explanation: At plait point, the separation factor becomes 1.
9. The plait point is the ____ tie line of the binodal curve.
a) Last
b) First
c) Middle
d) None of the mentioned
View Answer
Explanation: At plait point, the separation factor becomes 1 and both solubility curves merges.
10. Find the Distribution coefficient if equilibrium solute concentration in extract is 0.75 and the solute concentration in Raffinate is 0.6.
a) 1.25
b) 0.8
c) 1
d) 0
View Answer
Explanation: Distribution co-efficient = 0.75/0.6 = 1.25
11. The separation is possible if the solute concentration in both extract and raffinate are same.
a) True
b) False
View Answer
Explanation: At plait point, the extract and raffinate concentration are same so the separation is not possible.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Mechanical Engineering MCQs
- Practice Chemical Engineering MCQs
- Apply for Mechanical Engineering Internship
- Check Mechanical Engineering Books
- Apply for Chemical Engineering Internship
