This set of Basic Mass Transfer Questions & Answers focuses on “Solvent Selection for Unit Operations”.
1. For extraction, the distribution co-efficient should be greater that 1.
a) True
b) False
View Answer
Explanation: If the distribution co-efficient is greater than 1 the solvent requirement for the extraction operation will be low.
2. The viscosity should be high for the solvents using in extraction operation.
a) True
b) False
View Answer
Explanation: If the viscosity is high, the handling will be really tough so for better handling the viscosity should be low.
3. The Azeotropic composition of the solute makes the extraction process economical.
a) True
b) False
View Answer
Explanation: For the Azeotropic composition, additional solvent is required to alter the azeotropes formations.
4. Find the separation factor if the ratio of weight fraction of solute in the extract to raffinate is 0.75. Also given the ratio of dilutant in raffinate to extract is 0.5.
a) 1
b) 1.25
c) 1.5
d) 1.75
View Answer
Explanation: Distribution coefficient = the ratio of weight fraction of solute in the extract to raffinate * the ratio of dilutant in raffinate to extract
=0.75/0.5 = 1.5.
5. The separation is possible if the ratio of weight fraction of solute in extract to raffinate is 0.15. Also given the ratio of dilutant in raffinate to extract is 0.5.
a) True
b) False
View Answer
Explanation: Distribution co-efficient = the ratio of weight fraction of solute in extract to raffinate * the ratio of dilutant in raffinate to extract
= 0.15/0.5 = 0.3 < 1 (Separation is not possible)
6. In absorption operation, the solubility of gas should be high on the solvent.
a) True
b) False
View Answer
Explanation: If the gas solubility is more in a solvent, the amount of solvent requirement is less.
7. The solvent used in the extraction should be more volatile.
a) True
b) False
View Answer
Explanation: After obtaining the distillate, the extract has to be separated from solvent by means of distillation. Generally for distillation more volatile compound needs less heat to evaporate.
8. By analysing this extraction equilibrium triangle, find the solvent requirement range.
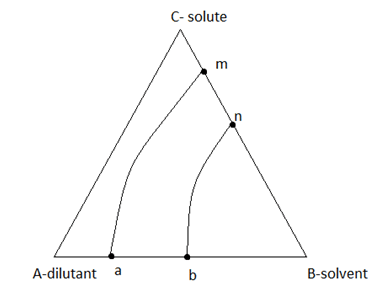
a) Less solvent
b) More solvent
c) No solvent
d) None of the mentioned
View Answer
Explanation: In this pair configuration, we need more solvent because the capacity to extract solute by the solvent is less.
9. For getting rapid absorption rate, low pressure drop and more heat transfer we need?
a) Low viscous solvent
b) More viscous solvent
c) No viscous solvent
d) None of the mentioned
View Answer
Explanation: Low viscous solvents are easier to handle and provide us low pressure drop etc.
10. In extract, the usage of solvent should be less.
a) True
b) False
View Answer
Explanation: If less solvent is used we can reduce the heat cost for separation by distillation in the extract phase.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice basic questions on all areas of Mass Transfer, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Mass Transfer Books
- Check Mechanical Engineering Books
- Practice Chemical Engineering MCQs
- Practice Mechanical Engineering MCQs
- Apply for Mechanical Engineering Internship
