This set of Mass Transfer Questions & Answers for experienced focuses on “Humidification-Vapour-Gas Mixture “.
1. Partial pressure equals vapour pressure if it is___________
a) Saturated
b) Unsaturated
c) Isothermal
d) None of the mentioned
View Answer
Explanation: At saturation, the vapour-gas mixture will be at equilibrium so the vapour pressure equals partial pressure.
2. Say True or False
I) Vapour- at superheated temperature
II) Gas – at condensation temperature
a) True
b) False
View Answer
Explanation: Since vapour has liquid portion we cannot say superheated. Superheated means free from other components like unique gas.
3. Guess the saturated molar absolute humidity of the gas(A.- Vapour(B. mixture at 100 degree Celsius. Vapour pressure of A is 300 mmHg and the total pressure is 760 mmHg.
a) 0.39
b) 0.65
c) 0.60
d) Cannot determine
View Answer
Explanation: Saturated molar humidity= Vapour pressure of A/ Vapour pressure of B
= 300/(760-300) =0.65.
4. The system is unsaturated if partial pressure _________ equilibrium vapour pressure.
a) Less than
b) Greater than
c) Equals to
d) All of the mentioned
View Answer
Explanation: If saturated, the vapour pressure equals partial pressure. If unsaturated, the pressure will be less than vapour pressure.
5. Consider a saturated gas(A.-vapour(B. mixture , if the absolute molal humidity is 0.265 and the total pressure= 760 mmHg. Find the vapour pressure of B.
a) 149.21 mmHg
b) 159.21 mmHg
c) 169.21 mmHg
d) 600.8 mmHg
View Answer
Explanation: First, we have to find the vapour pressure of A.
0.265= pA/760-pA
pA= 159.21
pB=760-159.21= 600.8.
6. The vapour pressure of the nitrogen gas is 400mmHg and the partial pressure is 300mmHg. Find the relative saturation.
a) 75
b) 133
c) 100
d) 50
View Answer
Explanation: Relative saturation or relative humidity = (partial pressure/vapour pressure. x100
=75.
7. Thermometer measurement temperature is
a) Dry bulb temperature
b) Wet bulb temperature
c) Dew point temperature
d) Room temperature
View Answer
Explanation: Dry bulb temperature is the actual measuring temperature of the thermometer.
8. Find the dew point
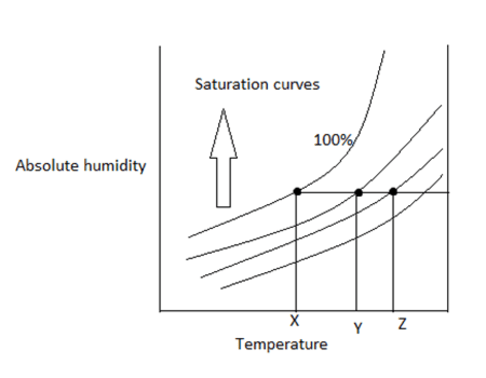
a) X
b) Y
c) Z
d) None of the mentioned
View Answer
Explanation: The point at 100% saturation is known as dew point. Below the point, the vapour starts to condense.
9. Temperature at which the partial pressure of the gas-vapour mixture equals vapour pressure _______
a) Dry bulb temperature
b) Wet bulb temperature
c) Dew point temperature
d) None of the mentioned
View Answer
Explanation: At saturation, the vapour pressure equals partial pressure. At dew point, saturation attains.
10. Check whether we get approximately 80% saturation if the total pressure and the partial pressure of the gas mixture is 760 mmHg and 260 mmHg. Also, the vapour pressure is 300 mmHg.
a) True
b) False
View Answer
Explanation: Percentage saturation is the ratio of absolute molal humidity to the saturated molal humidity. Absolute molal humidity= 260/(760-260. =0.52. Saturated molal humidity= 300/(760-300 =0.652.
%saturation= 0.52/0.652= 79.8(Approximately 80.
Sanfoundry Global Education & Learning Series – Mass Transfer.
To practice all areas of Mass Transfer for experienced, here is complete set of 1000+ Multiple Choice Questions and Answers
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Mass Transfer Books
- Apply for Mechanical Engineering Internship
