This set of Chemical Industry Multiple Choice Questions & Answers (MCQs) focuses on “Polymerization”.
1. Which of the following is not a method of classification of polymers?
a) Mode of preparation
b) Physico-chemical properties
c) Technical applications
d) Functional group
View Answer
Explanation: Functional groups are not used in the method of classification of polymers. Physico-chemical properties consider structure models for classification. Technical application is based on the end use of polymers, and mode of preparation distinguishes polymers on the basis of methodology used for the preparation of polymers.
2. Which of the following is the most used method for polymerization?
a) Bulk polymerization
b) Solution polymerization
c) Emulsion polymerization
d) Suspension polymerization
View Answer
Explanation: Emulsion polymerization is the most used method for polymerization, because it has an advantage of better heat control and it has very low degree of polymerization than other methods. Suspension polymerization is a heterogeneous radical polymerization process. Bulk polymerization is carried out by adding a soluble radical initiator to pure monomer in liquid state. In Solution polymerization a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.
3. Which of the following is not an optical property of polymer?
a) Luster
b) Dimensional stability
c) Reflectivity
d) Clarity
View Answer
Explanation: Dimensional stability is not an optical property of polymer. Optical property consists of luster, clarity, reflectivity, and permanent coloring. Dimensional stability is required to check the feasibility and stability of the reaction to occur.
4. Where is Castrol (India) Limited located?
a) Manali
b) Delhi
c) Mumbai
d) Chennai
View Answer
Explanation: Castrol India Limited is an automotive and industrial lubricant manufacturing company located in Mumbai. Castrol India is the 2nd largest manufacturer of automotive and industrial lubricants in the Indian lubricant market.
5. A polymeric chain cannot be built from acid alone.
a) True
b) False
View Answer
Explanation: To form a polymer, we need reactive bonds or groups available for coupling at the beginning or during the course of polymeric reaction. Single acid molecule can provide maximum one site after removal of H+ ion and to form a polymer we need multiple site. Only acid molecule cannot provide multiple reactive bond sites for formation of polymer.
6. The degree of polymerization indicates the Cis-trans position of a polymeric chain.
a) True
b) False
View Answer
Explanation: The degree of polymerization does not indicate the Cis-trans position of a polymeric chain. The degree of polymerization (DP or Xn) is defined as the number of monomer units in the polymer. It is calculated as the ratio of molecular weight of a polymer and molecular weight of the repeat unit.
7. Silicone monomers were first prepared by which method?
a) Condensation method
b) Grignard method
d) Bulk polymerization method
e) Co-polymerization method
View Answer
Explanation: Silicone monomers were first prepared by Grignard method, in which alkyl was substituted step by step for chlorine in SiCl4. The process is quite expensive and has been dropped in favor of direct method starting with silicon metal and alkyl.
8. Which of the following is not a semi synthetic polymer?
a) Cis-polyisoprene
b) Cellulose nitrate
c) Cellulose acetate
d) Vulcanized rubber
View Answer
Explanation: Cis-polyisoprene is not a semi synthetic polymer. Cis-polyisoprene is natural rubber. Cellulose nitrate, Cellulose acetate, and Vulcanized rubber are semi synthetic polymer because these are obtained from natural polymers.
9. Which of the following is not a characteristic of low density polythene (LDPE)?
a) Tough
b) Hard
c) Poor conductor of electricity
d) Highly branched structure
View Answer
Explanation: Hardness is not a characteristic of low density polythene (LDPE). Low density polyethene is flexible, branched, tough and poor conductor of electricity. Intermolecular forces (instantaneous-dipole induced-dipole attraction) are weaker in LDPE, due to which it not hard but more resilient.
10. Which of the following is an initiator in the free radical polymerization?
a) Bu-Li
b) AlCl3
c) HNO3
d) Benzoyl peroxide
View Answer
Explanation: Benzoyl peroxide acts as an initiator in the free radical polymerization. Due to the weak nature of the oxygen-oxygen bond in the peroxide group that connects the two C6H5CO groups, benzoyl peroxide tends to undergo homolytic fission.
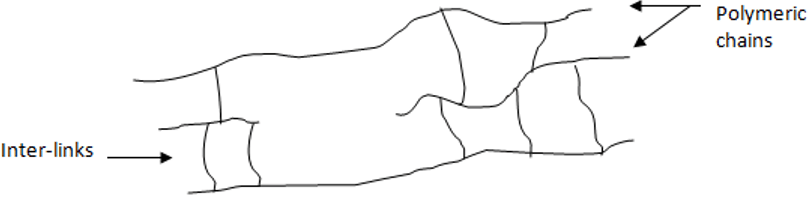
11. Which type of polymeric structure is depicted in the diagram given below?
a) Link chain polymer
b) Linear chain polymer
c) Cross-linked polymer
d) Branch chain polymer
View Answer
Explanation: In the given diagram we can clearly observe two three polymeric chains which are interconnected to each other with the help of cross-links which holds and strengthen the structure, only crosslink polymers have such structure and properties.
12. Which of the following is an elastomer?
a) Vinyls
b) Butadiene
c) Silicones
d) Polyamide
View Answer
Explanation: Butadiene is an elastomer. An elastomer is a resistant solid with high flexural strength. Vinyls are thermoplastic, Silicones are thermosetting polymer and Polyamides are classified in the series of fibers.
13. Duco cement is an example of which type of polymer?
a) Adhesive
b) Coating polymer
c) Fibers
d) Solid shape polymer
View Answer
Explanation: Duco cement is an example of an Adhesive. It’s a house hold adhesive that holds permanently on ceramic, glass, wood, leather, metal, paper, fabrics and nearly everything except on polyethylene or polypropylene plastics.
14. Which of the following is not a major classification of acrylic fiber?
a) Polyester
b) Polyamides
c) Polyolefins
d) Polyethylene
View Answer
Explanation: Polyethylene is not a major classification of acrylic fiber. It is an example of ethenic polymer under plastics whereas polyester, polyamides and polyethylene are the major classifications of acrylic fiber.
15. Molecules forming Polymers have weight ranges in which category?
a) Weight ranges between 104 – 106 grams
b) Weight ranges between 107 – 1010 grams
c) Weight ranges between 1010 – 1011 grams
d) Weight ranges between 1012 – 1017 grams
View Answer
Explanation: For the formation of polymers the required molecule size ranges from 104 to 106 grams. If we consider polymers having molecular weight more than the required range then it will be very difficult to break and mold molecules to form polymers and if we consider polymer of weight less than the required range it will require a huge amount of energy and resource to form polymers from them.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all areas of Chemical Industry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Process Technology Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
