This set of Chemical Industry Multiple Choice Questions & Answers (MCQs) focuses on “Inorganic Chemical – Nitric Acid”.
1. Which component is used as end application of nitric acid for production of toluene-diisocyanate?
a) 9% adipic acid
b) 3.5% dinitrotoluene
c) 3.5% nitrobenzene
d) 1% nitro -compounds
View Answer
Explanation: 9% adipic acid used in order to produce fiber and plastics precursor while 3.5% nitrobenzene is used for the production of aniline 1% nitro compounds for the production of explosives. Thus 3.5% dinitrotoluene is for the production of toluene-diisocyanate.
2. Which step mentioned below is not considered as fundamental step for the production of nitric acid?
a) Oxidation of NH3 to NO
b) Oxidation of NO to NO2
c) Oxidation of NH3 to NO2
d) Absorption of NO2 in water
View Answer
Explanation: All the steps including oxidation of NH3 to NO, oxidation of NO to NO2 and absorption of NO2 in water are the fundamental steps for the production of nitric acid.
3. Which process is involved in N2 fixation from air?
a) Wisconsin process
b) 2 NaNO3 + H2SO4 process
c) Ammonia oxidation process
d) Fission fragments process
View Answer
Explanation: In wisconsin process production of NO and NO2 by high temperature(2,200 C) reaction using air in gas-fired pebble bed heater followed by quick quench. In fission fragments process air exposed to radiation in a nuclear reactor to form NO.
4. Which process is used in order to concentrate nitric acid?
a) Concentration by H3PO4
b) Concentration by Mg(NO3)2
c) Concentration by Ca(NO3)2
d) Concentration by Ba(NO3)2
View Answer
Explanation: Magnesium nitrate solution containing 70-75% Mg(NO3)2 is fed to a dehydrating tray tower along with dilute HNO3 from the absorption system. The salt solution act as an extractive distillation agent, removing water at 100 C and the solution is again re- concentrated by evaporation.
5. Which are the factors favoured in the reaction kinetics in the NH3 oxidation stage?
a) High temperature, low pressure
b) Low temperature, high pressure
c) Low temperature, low pressure
d) High temperature, high pressure
View Answer
Explanation: In ammonia oxidation process, the reaction to form NO is favoured by increasing temperature until an optimum is reached which increases with higher gas velocities. Increasing pressure favours physical absorption rates and shifts chemical equilibrium to produce higher acid strengths.
6. Which promoter used in the platinum catalyst in production of nitric acid?
a) Alkali promoted
b) Metallic compounds promoted
c) Rh promoted
d) Arsenic promoted
View Answer
Explanation: Alkali and metallic compounds promoted promoters are used in the production of sulfuric acid. While alloying platinum with 2-10% rhodium promoted is used in order to improve yield at a given set of conditions in nitric acid production.
7. What is ‘X’ and ‘Y’ in the following flow chart?
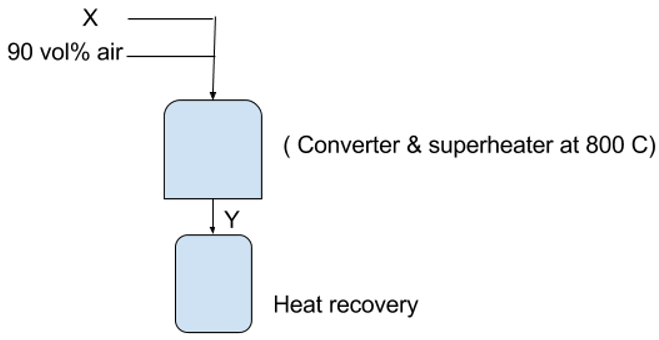
a) 10-12% NO, 10% NH3
b) 10% NH3, 10-12% NO
c) 10% NH3, 10-12% NO2
d) 10-12% NO2, 10% NH3
View Answer
Explanation: In nitric acid production, 90 vol % compressed air is mixed with 10 vol% anhydrous ammonia fed to a shell and tube converter, contain a steam heat recovery boiler- super heater at 800 C and gives output as producer gas containing 10-12% NO which is sent to heat recovery units.
8. In nitric acid concentration by H2SO4 which equipment is used for concentrating purpose?
a) Stoneware towers
b) Dehydrating tray towers
c) cooling towers
d) Chilling towers
View Answer
Explanation: In concentration by H2SO4 process, rectification by 93% can be obtain in silicon-iron or stoneware towers produces concentrated nitric acid and 75% concentrated H2SO4 which can be re-evaporated to 93%.
9. What is the major engineering problem associated with nitric acid production?
a) Complex heat balancing design
b) Furnace design
c) Thermodynamics and kinetic consideration
d) Materials of construction
View Answer
Explanation: Furnace design is the major engineering problem associated with production of phosphoric acid, while complex heat balancing is directly related to cryogenics. Thus, thermodynamics and kinetic consideration is associated with nitric acid production.
10. What is the disadvantage of using high pressure?
a) Lower oxidation yield
b) Higher acid strength
c) Higher oxidation yield
d) High reaction rates
View Answer
Explanation: Lower oxidation yield is one of the major disadvantages of using high pressure while others higher acid strength, high reaction rates are advantages of using higher process.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all areas of Chemical Industry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Check Chemical Process Technology Books
