This set of Chemical Industry Multiple Choice Questions & Answers (MCQs) focuses on “Inorganic Chemical – Fertilizer Industry”.
1. Which component of fertilizer is used in stimulates early growth purpose?
a) Phosphorus
b) Nitrogen
c) Potassium
d) Oxygen
View Answer
Explanation: Phosphorus stimulates early growth and accelerates seeding or fruit formation in later stages of growth while potassium is essential to development of the starches of potatoes and grains.
2. Potassium is used as fertilizer for which purpose?
a) Development of stems and leaves
b) Accelerating seeding
c) To prevent disease
d) Early stages of plant growth
View Answer
Explanation: Potassium is supplied in the soil to prevent disease and to lessen the effects of excessive nitrogen application. Accelerating seeding is done by phosphorus, early stages of plant growth and development of stems and leaves are done by nitrogen.
3. Which salt of potassium consist highest amount of Nacl?
a) Carnallite
b) Sylvinite
c) Hard salt
d) Langbeinite
View Answer
Explanation: Sylvinite contain 69.5% Nacl, carnallite contain 21.5% Nacl, hard salt contain 44.5% Nacl and 22.6% potassium chloride.
4. How liquid NH3 fertilizer can be produced?
a) By the gypsum process
b) By fauser process
c) By solvay process
d) From NH3 and HCL
View Answer
Explanation: Liquid NH3 fertilizer can be produced from gypsum process by double decomposition with ammonium nitrate. Solvay process and NH3-HCL are used to produce ammonium chloride which contains 24% nitrogen.
5. Which one is urea formaldehyde condensation fertilizer mentioned below?
a) Kalkammon
b) Lime urea
c) Cal-ka-nite
d) Uramite
View Answer
Explanation: Uramite is the urea formaldehyde condensation products which have been introducing as fertilizer. It is a condensation product of 3 moles of urea and 1 moles of formaldehyde. It consists of 38% nitrogen.
6. How potassium nitrate salt is obtained?
a) From a mixture of KCl and NaNO3
b) From a mixture of KCl and HNO3
c) From a mixture of KCl and NH4NO3
d) From a mixture of KCl and KNO3
View Answer
Explanation: Potassium nitrate salt is obtained by adding potassium chloride to hot solutions of sodium nitrate. After that sodium chloride got separated and KNO3 is crystallizes on cooling.
7. What is the organic raw material source of potash?
a) Hard coal
b) Caliche
c) Wool suint
d) Sea water
View Answer
Explanation: The sheep secretes part of the potash from the grass it eats into the wool with it’s sweat. Crood wool contains about 50% suint of which 20% consists of potassium salts and remainder of fats.
8. Which component is remain as ‘final liquor’ in KCl recovery process?
a) Nacl
b) K2O
c) K2SO4
d) Mgcl2
View Answer
Explanation: In KCl recovery process a high concentration of magnesium chloride solutions remain as ‘final liquor’ containing only small amount of alkali chloride. Mgcl2 is much more water soluble than KCl at low temperature.
9. From where K2SO4 is recovered?
a) Epsom salts
b) Hard salts
c) Baracite
d) Rock salt
View Answer
Explanation: K2SO4 is recovered from epsom salts in recovery of byproducts of potassium industry. Pure epsom salt is obtained by crystallization from concentrated solution in wooden vats.
10. What is ‘X’ in the following flow chart?
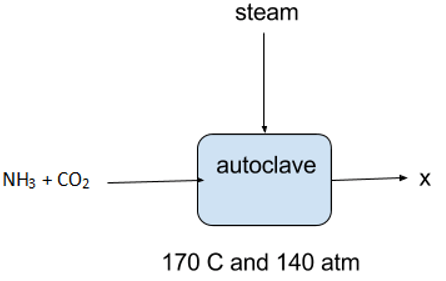
a) Urea
b) Ammonium sulfate
c) Ammonium chloride
d) Only ammonia
View Answer
Explanation: If a mixture of ammonia, carbon -di- oxide and steam is heated in an autoclave to about 170 C and 140 atm urea can be obtained from the intermediate ammonium carbonate.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all areas of Chemical Industry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Chemical Process Technology Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
