This set of Chemical Industry Multiple Choice Questions & Answers (MCQs) focuses on “Inorganic Chemical – Sulfuric Acid”.
1. What are the basic raw materials for manufacture of sulfuric acid?
a) Sulfur and pyrites
b) Oxygen
c) Hydrogen
d) Nitrogen
View Answer
Explanation: 85 to 95% sulfuric acid is produced by mining and commercial oxidization of 80 to 90% sulfur containing and 10 to 15% pyrates containing raw materials. Nitrogen is used as raw materials in the production of ammonia, nitric acid etc.
2. Which phenomena is involved in the byproduct sulfuric acid production?
a) Elemental sulfur mining
b) Oxidation -reduction
c) Pyrolysis of pyrites
d) Burning of fuel
View Answer
Explanation: Burning of fuel involves the production of waste gases which is used to produce sulfuric acid. This operation is known as byproduct sulfuric acid operation. Oxidation reduction and elemental sulfur mining is basically used to produced sulfur.
3. Which promoter mentioned below is used in the contact process?
a) Iron
b) Alkali
c) Acid
d) Air
View Answer
Explanation: In contact process alkali is used as promoter in trace amounts to enhance activity of catalytic agent in order to decrease the reaction time for better economy.
4. Which one of the following catalysts used in contact process before the using of vanadium pentoxide?
a) Aluminium
b) Zinc
c) Copper
d) Platinum
View Answer
Explanation: Platinum catalyst was previously used but suffers from easy poisoning, fragility, rapid heat deactivation and high initial investment. Except this other catalysts are not economical.
5. Which commercial product is produced by Frasch Process?
a) Sulfur
b) Ammonia
c) Nitric acid
d) Sulfuric acid
View Answer
Explanation: Sulfur is produced by Frasch process from salt domes as a result of elemental sulfur mining. Sulfuric acid produced by contact process and the nitric acid is produced by ammonia oxidation process.
6. What is 1, 2, 3 represents in the following flow chart in production of sulfur?
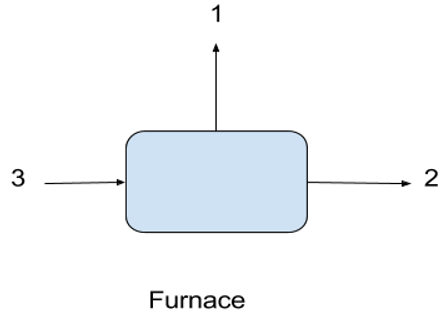
a) 1-steam, 2-H2S, 3-SO2
b) 1-H2S, 2-steam, 3-SO2
c) 1-steam, 2-SO2, 3-H2S
d) 1-SO2, 2-H2S, 3-steam
View Answer
Explanation: In the furnace H2S and air mixture burned by exothermic reaction and heat is liberated in the form of steam and two stage catalytic converter produced SO2 as final product from the furnace.
7. Which one of the following product is manufacturer by Finnish Process?
a) Elemental sulfur
b) Chlorine
c) Pyrrhotite
d) Sour gas
View Answer
Explanation: Elemental sulfur is produced by thermal dissociation and general combustion of pyrites in Finnish process and in general chlorine is produced by mercury cell electrolysis, diaphragm cell electrolysis and membrane cell electrolysis.
8. Which component of sulfur attacks the vanadium catalyst?
a) Platinum
b) Zinc
c) Arsenic
d) Lead
View Answer
Explanation: In the contact process arsenic usually attacks the vanadium catalyst. It can be removed by contacting molten sulfur with milk of lime in a continuous autoclave. Lead and Zinc usually don’t react with vanadium under the conditions provide in the contact process.
9. Which Major Engineering problem is associated with Frasch process?
a) Grinding
b) Corrosion
c) Final clean-up of stack gases
d) Pyrites ore beneficiation
View Answer
Explanation: Sulfur contain Shipment-insulated Barges and tank cars easily got corroded in the storage and handling process .Grinding and pyrites ore beneficiation are the major engineering problem of Finnish process, final clean-up of stock gas is related to oxidation reduction of H2S .
10. Why roasting of FeS is done?
a) Removal of arsenic
b) Recovery of H2S
c) Immune to poisons
d) Recovery of SO2
View Answer
Explanation: Roasting of FeS is done in Finnish process to recovery of SO2 from pyrrhotite. Removal of arsenic is done in order to protect vanadium by contacting molten sulfur with milk of lime in a continuous autoclave.
11. What is the name of the reactor used in the below flow chart?
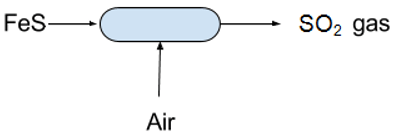
a) Hot reactor
b) Wash tower
c) Decomposition tower
d) Fluidized roaster
View Answer
Explanation: In the production of sulfur from pyrites Fluidized roaster is used to produce SO2 gas which is cooled in wash tower and waste heat boiler. Hot reactor is used to maintain the reaction temperature.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all areas of Chemical Industry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Chemical Process Technology Books
- Apply for Chemical Engineering Internship
