This set of Chemical Industry Questions and Answers for Freshers focuses on “Inorganic Chemical – Ammonia – 2”.
1. Which one is the highest end use for ammonia?
a) Urea
b) Nitric acid
c) Direct application as fertilizer
d) Acrylonitrile
View Answer
Explanation: The end uses for ammonia worldwide are as follows direct application as fertilizer- 25%, urea- 21%, nitric acid-12% and acrylonitrile – 3%.
2. What is the ratio of hydrogen and nitrogen used in the production of ammonia?
a) 3 moles pure H2 : 1 mole pure N2
b) 2 moles pure H2 : 2 moles pure N2
c) 1 moles pure H2 : 3 moles pure N2
d) 3 moles pure H2 : 3 moles pur N2
View Answer
Explanation: In ammonia synthesis, 3 moles of pure H2 and 1 mole of pure N2 is compressed to the operating pressure between 100 to 1000 atm. It is sent through a filter to remove compression oil.
3. What is the cooling agent use in the ammonia synthesis process?
a) Cooling water
b) Fuel gas
c) Cooling air
d) Cooling gas
View Answer
Explanation: The relatively cool gas is added along the outside of converter tube walls to provide cooling so that carbon steel can be used for thick wall pressure vessel and internal tubes.
4. Ammonia production is favoured by which following factor?
a) High temperature and low pressure
b) Low temperature and high pressure
c) Low temperature and low pressure
d) High temperature and high pressure
View Answer
Explanation: From the equilibrium standpoint this ammonia production reaction is favoured by low temperature and high pressure according to Le chatelier’s principle. At low temperature the rate of backward reaction will more than forward reaction.
5. Why iron oxide used in the ammonia production process?
a) To reduce porous iron
b) To increase porous iron
c) To maximize operating temperature
d) To maximize operating pressure
View Answer
Explanation: In ammonia production, preheated gas flows next through the inside of the tube which contains promoted porous iron oxide and this help to reduce porous iron in the start-up phases of operation in the synthesis reactor.
6. Which unit operation is not involved in the solvay process?
a) Saturation
b) Clarification
c) Liquefaction
d) Precipitation
View Answer
Explanation: In solvay process, saturated brine is pumped into saturator via a gas washer and saturated with ammonia and this saturated ammonia is pumped through clarifier in order to remove precipitated water hardeners.
7. In which process of ammonia production higher amount of conversion can be obtain?
a) Claude du point process
b) Casale
c) Haber
d) Kellogg
View Answer
Explanation: In claude du point process highest amount of conversion can be obtain which is 40-80%, haber and kellogg process gives 8-20% conversion while 15-25% conversion is given by casale ammonia production process.
8. In ammonia production process which factor increased the pumping cost?
a) High pressure vessel
b) Higher space velocity
c) Low pressure vessel
d) Lower space velocity
View Answer
Explanation: In ammonia production in addition to higher space velocity, cooling the bed increased the cost of NH3 recovery and pumping cost as well. Using of high pressure vessel increased the cost of reactor.
9. The fraction of ammonia in the exit gas depends on which factor?
a) Temperature
b) Pressure
c) Space velocity
d) Surface area
View Answer
Explanation: The fraction of ammonia in the exit gas depends on the space velocity. As the space velocity or flow rate increased the fraction of ammonia will get decreased.
10. What is ‘X’ in the following flow chart?
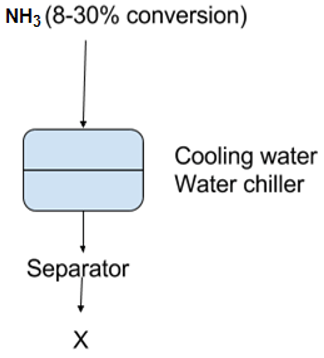
a) NH3 (85-90% yield)
b) N2
c) H2
d) N2 and H2
View Answer
Explanation: The NH3 product, with an 8-30% conversion is removed by condensation, first with water cooling and then NH3 refrigeration. The unconverted N2-H2 mixture is send to separator in order to produced 85-90% yield.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all areas of Chemical Industry for Freshers, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Chemical Process Technology Books
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
