This set of Chemical Industry written test Questions & Answers focuses on “Inorganic Chemical – Electrochemical Industries”.
1. What is the chlorine-caustic process in electrochemical industries?
a) Electrowinning
b) Electrorefining
c) Electro-separation
d) Electroplating
View Answer
Explanation: Electro-separation is related to the separation of chlorine-caustic mixture, electrorefining is the production of high purity copper from crude copper anodes, electrowinning is extraction of low purity copper from one leachings.
2. In which process alloy steels are produced?
a) Electric furnacing
b) Fused electrolytic
c) Gas phase electric discharge
d) Electroplating
View Answer
Explanation: In electric furnacing process, alloy steels are produced while aluminium, magnesium and sodium cells are related to fused electrolytic, ozone is related to gas phase electric discharge and nickel, chrome plating on steel are done in electroplating.
3. In which electrochemical cell, exothermic changes occur?
a) Electrolysis cell
b) Volatile cell
c) Fuel cell
d) Conventional cell
View Answer
Explanation: Electrolysis cell uses electrical energy to produce endothermic chemical changes, fuel cell and conventional cells provide endothermic change while volatile cell uses exothermic chemical reactions to produce electrical energy.
4. Which process of electrochemical industry consists of plasma?
a) Electro organic chemical process
b) Graphite production process
c) Arc process
d) Electrorefining
View Answer
Explanation: In arc process, electrons are released by thermionic emission at the cathode bombard the anode, causing heating and volatilization of anode material. The plasma consists of high velocity electrons, ions of the original gaseous media, plus anode and cathode material.
5. Which one mentioned below is not a chemical process for endothermic reaction?
a) Pyrolysis of hydrocarbon
b) Production of hydrogen cyanide
c) Reduction of oxides
d) Chlorine-caustic separation
View Answer
Explanation: Except chlorine-caustic separation process all are the mentioned above -pyrolysis of hydrocarbon, production of hydrogen cyanide and reduction of oxides are the chemical process where endothermic reactions occur.
6. What is arc image furnace?
a) Photochemical application of arc
b) Mechanical application of arc
c) Chemical application of arc
d) Photochemical conversions
View Answer
Explanation: Arc image furnace is the radiation and photochemical application of high intensity arc, while mechanical application of arc is such as new design of wind tunnels, cutting and welding of metals etc.
7. In which process low cost acrylonitrile is produced?
a) Arc process
b) Electro organic chemical process
c) Electrorefining
d) Electrowinning
View Answer
Explanation: Electro organic chemical process uses low-cost acrylonitrile made in one step from propylene, ammonia and air to give adiponitrile in a second step. Alternatively, one must go in four steps from benzene to cyclohexane to adipic acid to adiponitrile.
8. Which raw material mentioned below is not used in the production of tetraethyl-Lead?
a) Grignard reagent
b) Ethyl chloride
c) Lead metal
d) Ethyl alcohol
View Answer
Explanation: Ethyl magnesium chloride (Grignard reagent), ethyl chloride and lead metals are the necessary raw materials for the production of tetraethyl-lead where lead metal is wholly consumed and need to be recycled.
9. How decomposition efficiency is defined?
a) Ratio of theoretical voltage to actual
b) Ratio of theoretical current to actual (one gm equivalent)
c) Ratio of equivalents produced to equivalents charged
d) Ratio of reversible voltage to actual
View Answer
Explanation: Decomposition efficiency is defined as the ratio of equivalents produced to equivalents charged while ratio of theoretical voltage to actual is introduced as voltage efficiency.
10. What is the output in the following process?
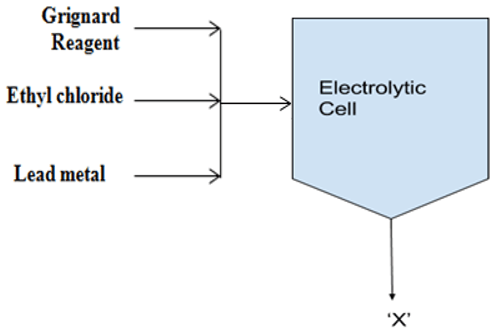
a) Tetraethyl lead
b) Ethyl lead
c) Diethyl lead
d) Triethyl lead
View Answer
Explanation: The electrolytic cell uses a sacrificial lead anode and a stainless-steel cathode. Electrolyte comprises the Grignard reagent with ethyl chloride and later lead metals are added to the cell in the form of pellets to produce tetraethyl lead.
Sanfoundry Global Education & Learning Series – Chemical Industry.
To practice all written questions on Chemical Industry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Check Chemical Process Technology Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
