This set of Materials Science Multiple Choice Questions & Answers (MCQs) focuses on “Eutectic Systems”.
1. What is the line that defines the solubility limit of A in B and B in A from the figure?
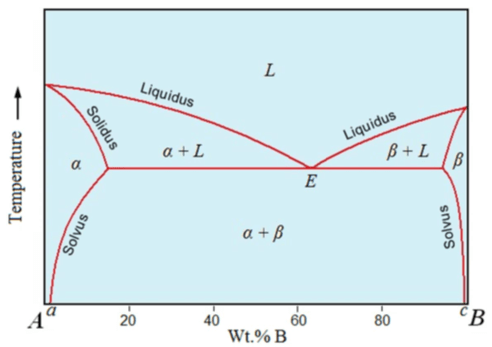
a) Solidus line
b) Liquidus line
c) Solvus line
d) Solidus line and Liquidus line
View Answer
Explanation: In addition to liquidus and solidus lines there are two more lines on A and B which define the solubility limits B in A and A in B respectively. These are called solvus lines.
2. What is the point at which three phases (L+α+β) coexist at point E?

a) Peritectic point
b) Eutectic point
c) Eutectoid point
d) Peritectoid reaction
View Answer
Explanation: Eutectic reaction is the reaction in which the Liquid phase directly converts into two different solid phases at a constant temperature known as eutectic temperature.
3. The micro structure of hypoeutectic alloys at room temperature consists of ________

a) Proeutectic β and α
b) Eutectic mixture (α+β)
c) Proeutectic β and eutectic mixture (α+β)
d) Proeutectic α and eutectic mixture (α+β)
View Answer
Explanation: In hypoeutectic alloys the αphase solidifies first and the micro structure at RT consists of this αphase (called proeutectic α) and the eutectic (α+β) mixture.
4. Why Pb-Sn eutectic alloys are used for soldering purpose?
a) The melting point at eutectic point is maximum
b) The melting point at the eutectic point is minimum
c) The melting point at the eutectic point is constant
d) The boiling point at the eutectic point is maximum
View Answer
Explanation: The melting point at the eutectic point is minimum. That’s why Pb-Sn eutectic alloys are used as solders. Al-Cu, Al-Si, Ag-Cu are other eutectic systems.
5. Crystals of which material begin to form at point a from the figure?
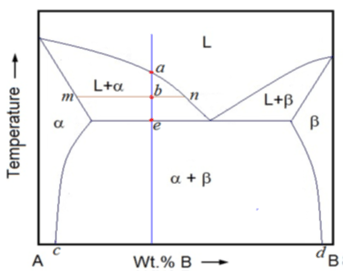
a) Crystals of proeutectic α
b) Crystals of proeutectic β
c) Crystals of eutectic (α+β)
d) Crystals of proeutectic α and β
View Answer
Explanation: While cooling a hypoeutectic alloy which is in a liquid state, the temperature drops continuously till liquidus point, a, at which crystals of proeutectic αbegins to form.
6. At point b, the α (alpha) fraction is given by the lever rule as ________

a) mn/bn
b) bn/mn
c) ab/be
d) be/ab
View Answer
Explanation: On further cooling the fraction of αincreases. At any point, b, in the two-phase region the α fraction is given by the lever rule as bn/mn.
7. The inflection in the cooling curve between points a and e is due to _________
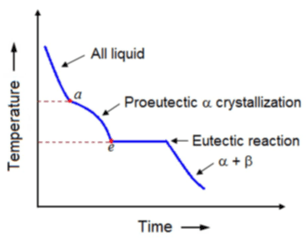
a) Latent heat
b) Low temperature
c) High temperature
d) Specific heat
View Answer
Explanation: Solidification of proeutectic αcontinues till the eutectic temperature is attained. The inflection in the curve between points a and e is due to latent heat.
8. At the eutectic point (e) the eutectic reaction proceeds at a constant temperature as ________

a) F = 1
b) F = 0
c) F = 2
d) F = 3
View Answer
Explanation: At the eutectic point the solidification of eutectic mixture (α+β) begins through the eutectic reaction which proceeds at a constant temperature as F = 0 (2 – 3 + 1).
9. Any composition left of point c or right of point d will cool and solidify like a _______

a) Eutectic
b) Proeutectic
c) Eutectoid
d) Isomorphous system
View Answer
Explanation: Any composition left of point c or right of point d (α and β single phase region respectively) will cool and solidify like an isomorphous system.
10. A 34.6% Pb-Sn alloy is cooled just below the eutectic temperature (183°C). What is the fraction of proeutectic α and eutectic mixture (α+β)?
a) 70% and 30%
b) 64% and 36%
c) 36% and 64%
d) 30% and 70%
View Answer
Explanation: The eutectic point is at 61.9% Sn and α boundary is at 19.2% Sn. Apply the lever rule % proeutectic α = 100*(61.9 – 34.6)/(61.9 – 19.2) = 64%
% (α + β) = 100*(34.6 – 19.2)/(61.9 – 19.2) = 36%.
Sanfoundry Global Education & Learning Series – Materials Science.
To practice all areas of Materials Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
