This set of Materials Science test focuses on “Diffusion Mechanisms – II”.
1. In steady state diffusion, which of the following remain unchanged with time?
a) Concentration at source
b) Concentration at sink
c) Concentration profile
d) All of mentioned
View Answer
Explanation: In steady state diffusion, the concentration of the diffusing species is kept constant at both ends and hence, the concentration at different points remain unaffected as time passes. Purification of hydrogen gas employs steady-state diffusion through a thin palladium sheet.
2. For steady-state diffusion, diffusion flux is proportional to the concentration gradient. Concentration gradient is:
a) Rate of change of concentration with respect to space
b) Rate of change of concentration with respect to time
c) Difference in concentrations at the source and sink
d) Ratio of concentrations at source and sink
View Answer
Explanation: Concentration gradient is the major driving force behind diffusion. It is the slope of the concentration profile and measures the difference in concentration of the diffusing species per unit length.
3. Which of the following is not true for steady-state diffusion?
a) The concentration profile is linear
b) The concentration gradient is constant
c) There is no net transfer of mass
d) The diffusion flux is constant
View Answer
Explanation: Although, the concentration at each point in the diffusion medium remains constant, yet the diffusing species is being transferred from a source end to sink end.
4. Concentration of hydrogen gas across a 2mm thick palladium sheet differs by 4 kg/m3. Considering steady-state diffusion with diffusion constant 10-10 m2/s, diffusion flux is ______ kg/m2.s:
a) 2 x 1010
b) 2 x 10-10
c) 5 x 10-11
d) 5 x 109
View Answer
Explanation: Magnitude of diffusion flux for steady-state diffusion is given by the product of diffusion constant and concentration gradient.
5. The expression J = M/(A.t), where J, M, A, & t are diffusion flux, mass of diffusing species, cross sectional area, and time respectively is:
a) incorrect
b) valid for steady-state diffusion only
c) valid for non steady state diffusion only
d) valid for both steady and non steady state diffusion
View Answer
Explanation: It follows directly from the definition of diffusion flux. It is the mass being transferred per unit area per unit time.
6. If C is the concentration of the diffusing species at distance x from the source and time t, then according to Fick’s second law (assuming diffusion coefficient, D is constant):
a) ∂C/∂x = D.(∂C/∂t)
b) ∂C/∂t = D.(∂2C/∂x2)
c) ∂C/∂x = D.(∂2C/∂t2)
d) ∂C/∂t = D.(∂C/∂x)
View Answer
Explanation: According to Fick’s second law, the rate of change of concentration of diffusing species with time at a given point in space is proportional to the slope of the concentration gradient curve at that point.
7. The relation between temperature and diffusion coefficient is:
a) Linear
b) Exponential
c) Sinusoidal
d) Diffusion coefficient is not related to temperature
View Answer
Explanation: Diffusion coefficient is indicative of rate of diffusion. Its relation with temperature is given by the expression: D = D0.exp( -Qd / R.T)
where D0 is diffusion coefficient at initial temperature, Qd is the activation energy, R is gas constant, and T is the change in temperature.
8. Predeposition and drive-in diffusion are two heat treatments used in the manufacture of integrated circuits.
Assertion: Predepostion is carried at a higher temperature than drive-in diffusion.
a) True
b) False
View Answer
Explanation: Drive-in diffusion is used to push the already added (through predeposition step) impurity atoms farther into the Si lattice to gain the desired impurity distribution and is carried out at approximately 1200℃ (contrary to 900℃ of predeposition).
9. Following plot shows the concentration profile at two different time, T1 and T2 for the same non-steady diffusion process.
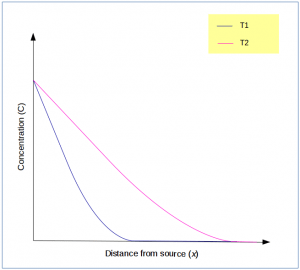
What is the correct relationship between the two?
a) T1 > T2
b) T2 > T1
c) Insufficient data
d) Same process can’t have two concentration profiles
View Answer
Explanation: As more time passes, the diffusing species reaches farther into the solid and tends to approach a linear shape.
10. Diffusion through grain boundaries is very fast and is called short-circuit diffusion.
Assertion: Their contribution to overall diffusion is negligible.
a) True
b) False
View Answer
Explanation: Due to very small cross sectional areas of the grain boundaries, the actual amount of mass transferred through them is much smaller than that through the bulk.
Sanfoundry Global Education & Learning Series – Materials Science.
To practice all areas of Materials Science for tests, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Material Science Books
- Check Metallurgical Engineering Books
- Practice Metallurgical Engineering MCQs
- Check Mechanical Engineering Books / Chemical Engineering Books
- Apply for Metallurgical Engineering Internship
