This set of Materials Science Multiple Choice Questions & Answers (MCQs) focuses on “Amorphous Solids”.
1. Which of the following properties is generally exhibited by amorphous solids?
a) Anisotropy
b) Glass-transition
c) Equal strength of all bonds
d) All of the mentioned
View Answer
Explanation: Due to random organization of particles, amorphous solids have the same physical properties along all directions, or are isotropic. Random organization of particles also results in unequal bond strengths. Upon cooling, amorphous solids turn into a brittle glass-like state from a flexible rubber-like state. This is called glass-transition.
2. Metal glass was first prepared at:
a) California Institute of Technology
b) Massachusetts Institute of Technology
c) Technion
d) University of Michigan
View Answer
Explanation: Metal glass or amorphous metal was reportedly first produced by W. Klement (Jr.), Willens and Duwez in 1960 at Caltech. It was prepared by rapid cooling (~ 1 MK/s) of molten metal alloys.
3. Polycrystalline solids are isotropic.
a) True
b) False
View Answer
Explanation: Anisotropy is a characteristic behavior shown by ideal crystals. However, the presence of flaws like grain boundaries causes the solid to deviate from crystalline properties.
4. Metal glasses differ from their crystalline counterparts in many ways. Chief application(s) of metal glasses include(s):
a) Bullet-proof glasses
b) Power transformers
c) Conducting wires
d) All of the mentioned
View Answer
Explanation: Amorphous metals are not transparent and have relatively lower electrical conductivity. However, most metal glasses possess high magnetic susceptibility and low coercivity.
5. Consider the following cooling diagram for an amorphous solid.
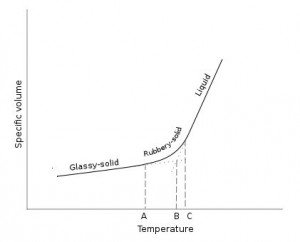
Glass-transition temperature is represented as:
a) A
b) B
c) C
d) None of the mentioned
View Answer
Explanation: An exact melting point does not exist for amorphous solids. An approximate glass-transition temperature is defined by extrapolating the cooling curve as shown.
6. Soda-lime glass is the most common type of glass. The component present in largest w/w percentage is:
a) SiO2
b) Al2O3
c) Na2O
d) CaO
View Answer
Explanation: Glass inherits its transparency from crystalline SiO2, also called quartz. However, quartz has very high and narrow glass-transition. To overcome this, small amount of Na2 is added. CaO is also added to prevent water-solubility imparted by soda.
7. Lead-oxide glass is called “crystal glass” because:
a) It contains crystalline Pb
b) It contains SiO2 crystals
c) It contains PbO crystals
d) None of the mentioned
View Answer
Explanation: Well, crystal glass is amorphous, not crystalline. This glass earns its name from its excellent decorative properties and high refractive index.
8. Crystallinity increases with increasing rate of cooling of a liquid.
a) True
b) False
View Answer
Explanation: When a liquid is cooled rapidly, the particles get less time to move and arrange themselves in an orderly fashion and hence the crystallinity of the resulting solid decreases.
Sanfoundry Global Education & Learning Series – Materials Science.
To practice all areas of Materials Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Mechanical Engineering MCQs / Chemical Engineering MCQs
- Apply for Mechanical Engineering Internship / Chemical Engineering Internship
- Practice Metallurgical Engineering MCQs
- Apply for Metallurgical Engineering Internship
- Check Metallurgical Engineering Books
