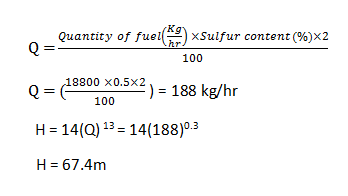This set of Energy Engineering Quiz focuses on “Draught System – 2”.
1. Which is the net pressure equation used to find chimney height?
a) P = H (Wa-Wg)
b) P = H (Wa+Wg)
c) P = H (Wg-Wa)
d) P = H (\(\frac{W_a}{W_g}\))
View Answer
Explanation: The pressure acting on the grate from chimney side,
P1 = Pa + Wg H
Pressure acting on the grate from atmospheric side
P2 = Pa + Wa H
Where, Pa = Atm pressure
Wa = weight density of air
Wg = Weight density of hot gases
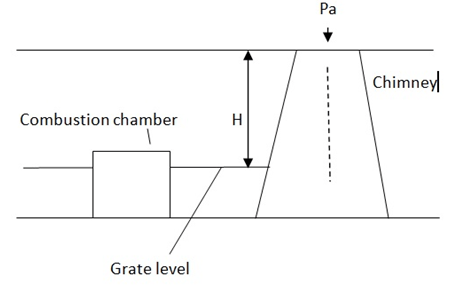
The net pressure acting on the combustion chamber due to the pressure exerted by gas column and air column is given by
P = P2 – P1 (as Wa > Wg )
P = (pa + Wa H) – (Pa + Wa H)
P = H (Wa – Wg )
This pressure difference is known as static draught and is responsible for causing the flow of air through the chimney.
2. Which is the equation used to find chimney diameter?
a) D = 1.128\(\sqrt{\frac{Mg}{ρg V}}\)
b) D = 5.48\(\sqrt{\frac{ρg V}{Mg}}\)
c) D = 5.48\(\sqrt{\frac{Mg}{ρg V}}\)
d) D = 1.128\(\sqrt{\frac{ρg V}{Mg}}\)
View Answer
Explanation: The mass of gases flowing through any cross section of the chimney is given by
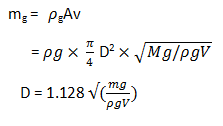
3. The portion of flue gases carried away to produce draught could be utilized to _______
a) Heat the air entering furnace
b) Blow out the combustion products such as soot and ash
c) Heat the fuel in ash chamber
d) Support the combustion
View Answer
Explanation: It is evident that the draught is created at the cost of thermal efficiency of boiler plant installation since a portion of flue gases carried away by the flue gases to produce the required draught could have been used either in heating the air entering the furnace or in heating the feed water, which would increase the thermal efficiency.
4. Determine the height of chimney above grate level. Where diameter of chimney is 1.75m and produces a draught of 1.8cms of water. Temperature of flue gases is 290oC. The flue gases formed per kg of fuel burnt are 23kg. Neglect the losses and assume atmospheric temperature as 20oC?
a) H = 23.28m
b) H = 18.56m
c) H = 32.77m
d) H = 41.92m
View Answer
Explanation: Given: D = 1.75m,
Draught in mm of water = 1.8cm = 18mm
Flue gas temperature = Tg = 290oC = 563K
Ambient temperature = Ta = 20oC = 293k
Flue gases formed per kg of fuel burnt: (ma + 1) = 23kg
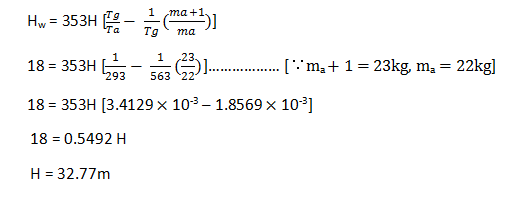
5. A draught of 15mm of water is produced by using a chimney of height 30m. The ambient air and flue gases are at 27oC and 300oC respectively. The coal burned on the grate contains 81% carbon, 5% moisture and remaining ash. Neglect the losses and assume values of burnt products equivalent to the volume of air supplied and for the complete combustion of fuel, find the percentage of excess of air supplied?
a) 14.56%
b) 52.89%
c) 8.13%
d) 20.002%
View Answer
Explanation: Given: Draught in mm of water = 15mm
Height of chimney = 30m
Ambient temperature, Ta = 27 + 273 = 300K
Flue gas temperature, Tg = 300 + 273 = 573K
% of carbon = 81%
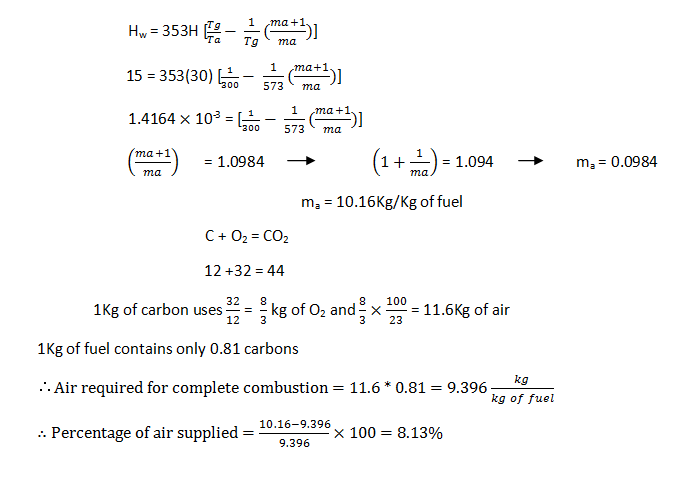
6. Determine the height of the chimney to produce a static draught of 22mm of water if the mean flue gas temperature in chimney is 290oC and ambient temperature in boiler house is 20oC. The gas constant for air is 29.26Kgm/Kgk and for chimney flue gas is 26.2 Kgfm/Kgk. Assume reading as 760mm of mercury?
a) 38.42m
b) 42.55m
c) 45.84m
d) 44.03m
View Answer
Explanation: Density of air at 290K ρg = P/RT = [1.033 * 104 / 29.26 * 290] = 1.217Kg/m3
Density of fuel gas at 563K, ρg = [1.033 * 104 / 26.2 * 563] = 0.7 Kg/m3
Static Draught, p = H (ρa– ρg)
22 = H (1.217 – 0.7)
H = 42.55m
7. The flue gases of natural draught are at higher temperature when compared to flue gases in artificial gas.
a) True
b) False
View Answer
Explanation: The flue gases of natural draught are at higher temperature when compared to flue gases of artificial gas because to maintain certain minimum temperature required to produce a given draught for the given height of the chimney. Due to higher flue gas temperature, the heat lost with flue gases is more in natural draught.
8. Determine the height of the chimney to get net draught of 12mm if the total losses are 4mm. the temperature of air is 25oC and the temperature of chimney gases is 300oC. The mass of air used per kg of fuel used is 18kg. One kg of air occupies a volume of 0.7734m3 at normal temperature?
a) 26.84
b) 20.22
c) 29.93
d) 18.09
View Answer
Explanation: Density of air at normal temperature = 1/0.7734
= 1.293Kg/m3
Density of air at 298K, ρa = 1.293 × 273/298
= 1.1845 Kg/m3
Density of gases at 573K, ρg = 1.293 × ((18+1)/18) × 273/573
= 0.65 Kg/m3
But, P = 12 + 4
= 16 mm of water
P = H (ρa – ρg)
16 = H (1.1845 – 0.65)
H = 29.93m
9. A 15kg of air is supplied per kg of fuel burnt to the combustion chamber of a boiler using fuel 600kg/hr. the temperature of flue gases and ambient air are 273°C and 32°C. If the minimum draught required to start the flue is 9.5mm of water, find out the minimum height of the chimney?
a) 22.66m
b) 23.84m
c) 24.52m
d) 25.16m
View Answer
Explanation: Temperature of fuel gases Tg = 237 + 273 = 510K
Temperature of ambient air Ta = 32 + 273 = 305K
Drought in mm of water Hw = 9.5mm
Mass of air mg = 15kg
We have,
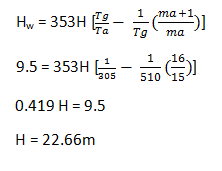
10. Using which of the given formula the chimney height is calculated to get the answer of 67.4m when the coal burnt is 18.8TPH and considering 0.5% of sulfur content in coal?
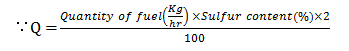
a) H = 12(Q)13
b) H = 17(Q)13
c) H = 14(Q)13
d) H = 22(Q)13
View Answer
Sanfoundry Global Education & Learning Series – Energy Engineering.
To practice all areas of Energy Engineering for Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Environmental Engineering MCQs
- Check Mechanical Engineering Books
- Check Environmental Engineering Books
- Apply for Environmental Engineering Internship
- Practice Mechanical Engineering MCQs