This set of Physical Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Quantum Theory – Angular Momentum”.
1. What is the angular momentum operator (Lz) in spherical coordinates?
a) Lz = -iħ(\(\frac{\partial}{\partial\phi}\) + r sin θ)
b) Lz = -iħ(\(\frac{\partial}{\partial\phi}\) + r cos θ \(\frac{\partial}{\partial\theta}\))
c) Lz = -iħ \(\frac{\partial}{\partial\phi}\)
d) Lz = -iħ \(\frac{\partial}{\partial\theta}\)
View Answer
Explanation: Purely in terms of differential operators, Lz = -iħ (x\(\frac{\partial}{\partial y}\) – y\(\frac{\partial}{\partial x})\). Plugging in the spherical coordinates conversion from Cartesian coordinates gives Lz = -iħ \(\frac{\partial}{\partial\phi}\). This is the reason why angular momentum is easier defined by its projection on the z-axis as it has the simplest expression amongst all other projections.
2. What are the eigenvalues for z component of angular momentum operator Lz? (m = magnetic quantum number)?
a) Lz = mħ
b) Lz = \(\frac{\hbar}{2\pi m}\)
c) Lz = mħ2
d) Lz = 0
View Answer
Explanation: Operating on spherical harmonics on Lz: Lz\(Y_m^l\) (θ, Φ) = mħ\(Y_m^l\)(θ, Φ), which yields eigenvalues of Lz = mħ for a given quantum mechanical system.
3. How many values of m (magnetic quantum number) in terms of l (angular quantum number)?
a) m = l
b) m = 2l
c) m = 2l+1
d) m = l(2l+1)
View Answer
Explanation: m can take values from -l to +l. Summing this up gives 2l + 1 values of m to operate with.
4. Consider a system that has the wavefunction φ =N sinΦ. What is the average angular momentum projection in the z-direction?
a) ħ
b) 3πNħ2
c) Nħ2
d) 0
View Answer
Explanation: Lz = -iħ\(\frac{\partial}{\partial\phi}\) = N2 \(\int_0^{2\pi}\) sinΦ (-iħ \(\frac{\partial}{\partial\phi}\) sin Φ) dΦ = N2 \(\int_0^{2\pi}\) sin Φ cos ΦdΦ = 0 (since its an odd function).
5. Consider a system that has the wavefunction φ = N cosΦ. What is the kinetic energy of rotation E about the z axis in terms of moment of inertia I?
a) E = ħ2I
b) E = \(\frac{\hbar^2}{2I}\)
c) E = 2ħ2I
d) E = 0
View Answer
Explanation: z axis kinetic energy is given by: \(\frac{1}{2I} L_z^2 = \frac{\hbar^2}{2I} \frac{d^2}{d\phi^2}\) —→ \(\frac{1}{2I} L_z^2\) cos Φ = \(\frac{\hbar^2}{2I}\) cos Φ = Ez cosΦ. This gives E = \(\frac{\hbar^2}{2I}\)
6. What is the expression for moment of inertia I in terms of reduced mass μ and internuclear distance Re?
a) I = \(\frac{R_e^2}{2\mu}\)
b) I = μ\(R_e^2\)
c) I = \(\frac{\mu}{R_e}\)
d) I = 2πμ\(R_e^2\)
View Answer
Explanation: In classical mechanics, moment of inertia I is defined as I = mR2; replacing this with reduced mass and internuclear distance gives I = μ\(R_e^2\).
7. What is reduced mass of HCl in kg? (H = 1, Cl = 35)?
a) 6.23×10-10 kg
b) 2.55×10-20 kg
c) 3.23×10-25 kg
d) 1.63×10-27 kg
View Answer
Explanation: μ = \(\frac{(1×10^{-3}kgmole^{-1})(35×10^{-3}kgmole^{-1})}{((1+35)×10^{-3})(6.023×10^{-23}mole^{-1})}\) = 1.626 × 10-23 ∼ 1.63 × 10-27 kg
8. What is the moment of inertia of HCl? (internuclear distance is 127.5 pm)?
a) 2.644 × 10-47 kgm2
b) 7.843 × 10-27 kgm2
c) 4.321 × 10-20 kgm2
d) 9.363 × 10-15 kgm2
View Answer
Explanation: I = μ\(R_e^2\) = \(\frac{(1×10^{-3}kgmole^{-1})(35×10^{-3}kgmole^{-1})}{((1+35)×10^{-3})(6.023×10^{-23}mole^{-1})}\) × (127.5 × 10-12m)2 = 2.644 × 10-47kgm2
9. What is the value of L for J=1?
a) 1.491 × 10-34Js
b) 9.322 × 10-25Js
c) 4.391 × 10-20Js
d) 8.273 × 10-26Js
View Answer
Explanation: L = \(\sqrt{J(J+1)}\)ħ = √2ħ = \(\frac{\sqrt 2(6.626×10^{-34} Js)}{2\pi}\) = 1.491 × 10-34Js
10. What is the value of E for an HCl molecule for J = 1?
a) 1.088 × 10-22J
b) 3.042 × 10-20J
c) 6.720 × 10-18J
d) 4.206 × 10-22J
View Answer
Explanation: I = μ\(R_e^2\) = \(\frac{(1×10^{-3}kgmole^{-1})(35×10^{-3}kgmole^{-1})}{((1+35)×10^{-3})(6.023×10^{-23}mole^{-1})}\) × (127.5 × 10-12m)2 × (127.5 × 10-12m)2 = 2.644 × 10-47kgm2. E = \(\frac{\hbar^2}{2I}\) J(J + 1) = \(\frac{(6.626×10^{-34})(2)}{8\pi^2×2.644×10^{-47}}\) = 4.206 × 10-22J
11. The diagram below shows projections of angular momentum onto the z-axis. What is the value of l (angular momentum quantum number)?
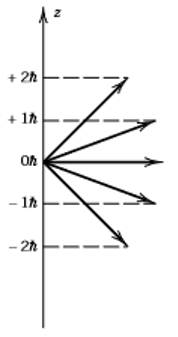
a) l = 0
b) l = 1
c) l = 2
d) l = -2
View Answer
Explanation: Since values of m range from +2h to -2h, the value of l must be 2 to hold these magnetic quantum numbers.
12. The diagram below shows projections of angular momentum onto the z-axis. What is the maximum value of the magnetic quantum number m?
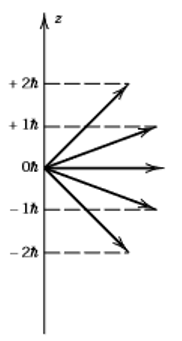
a) 1
b) 0
c) 2
d) -1
View Answer
Explanation: In the given quantum state of l = 2, m can take values from -2 to +2. Although it can take values of -1, 0, and 1, 2 is the maximum value of the magnetic quantum number.
13. What is the reduced mass of KCl in kg? (K = 39, Cl = 35)?
a) 3.06 × 10-26 kg
b) 2.02 × 10-18 kg
c) 9.23 × 10-24 kg
d) 4.11 × 10-20 kg
View Answer
Explanation: μ = \(\frac{39 × 35}{(39 + 35)}\) × 1.66 × 10-27 = 3.06 × 10-26 kg
14. What is the reduced mass of a hydrogen molecule?
a) 9.23 × 10-25 kg
b) 3.06 × 10-26 kg
c) 8.3 × 10-28 kg
d) 2.30 × 10-27 kg
View Answer
Explanation: μ = \(\frac{1 × 1}{(1 + 1)}\) × 1.66 × 10-27 = 8.3 × 10-28 kg
15. What is the reduced mass of carbon monoxide? (C = 12, O = 16)?
a) 3.33 × 10-25 kg
b) 4.23 × 10-22 kg
c) 9.01 × 10-24 kg
d) 1.14 × 10-26 kg
View Answer
Explanation: μ = \(\frac{12 × 16}{(12 + 16)}\) × 1.66 × 10-27 = 1.14 × 10-26 kg
Sanfoundry Global Education & Learning Series – Physical Chemistry.
To practice all areas of Physical Chemistry, here is complete set of Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
