This set of Biophysics Multiple Choice Questions & Answers (MCQs) focuses on “Thin Layer Chromatography”.
1. Which of the following mobile phase is used to separate hydrocarbons through TLC?
a) Butanol/ Acetic Acid/Water (80/20/20)
b) Hexane/ Chloroform/ Acetic acid (50/15/5)
c) 96% Ethanol/water (70/30)
d) Ethyl Acetate/ propanol (65/35)
View Answer
Explanation: The solvent with Butanol/ Acetic Acid/Water (80/20/20) is used to separate amino acids. The solvent with Hexane/ Chloroform/ Acetic acid (50/15/5) is used to separate hydrocarbons. The solvent with 96% Ethanol/water (70/30) is also used to separate amino acids. The solvent with Ethyl Acetate/ propanol (65/35) is used to separate mono and disaccharides.
2. Alkaline Tetrazolium is used to identify unknown amino acid compounds.
a) True
b) False
View Answer
Explanation: No, Ninhydrin reagent is used to identify unknown amino acid compounds. Alkaline Tetrazolium is used to identify corticosteroid compounds.
3. You had some unknown aromatic hydrocarbon samples. After perfectly performing TLC you did not observe any spot or band in the TLC plate. What could be the reason?
a) The mobile phase didn’t work properly
b) As hydrocarbons are volatile, so all the hydrocarbons got evaporated after I made the spots
c) There is a problem in stationary phage
d) Hydrocarbon spots are not visible to the naked eye
View Answer
Explanation: Hydrocarbons are volatile and absorb a very little amount in the spot. So after performing TLC, these spots are not visible to the naked eye due to having the same color as the solvent. We do expose the plate to UV light to detect spots of hydrocarbon.
4. What is used to detect hydrocarbon spots in TLC place with silica gel?
a) UV light exposure
b) Ninhydrin test
c) KMnO4
d) Alkaline Tetrazolium
View Answer
Explanation: UV light exposure is used to detect hydrocarbon spot in TLC place with silica gel. The hydrocarbons absorb short wavelength UV light but the solvent doesn’t, so hydrocarbons can be visible to UV light.
5. The following image shows a standard TLC plate for hydrocarbon separation. Detect the compound D.
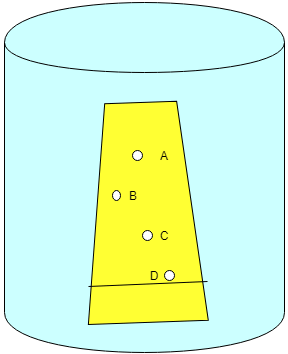
a) Naphthoic acid
b) Salicylic acid
c) Catechol
d) Xylene
View Answer
Explanation: The structure of catechol is
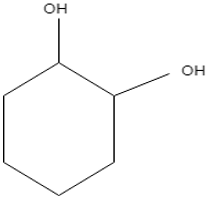
Due to the presence of two highly electronegative – OH groups in Ortho-position, catechol is a very polar molecule. Now, in standard TLC experiment for hydrocarbon separation, a nonpolar solvent is used. Therefore, catechol moves the least distance because of high polarity.
6. The fluorescence of Salicylic acid can be observed by placing the TLC place under UV light of __________ nm.
a) 302
b) 205
c) 150
d) 400
View Answer
Explanation: The UV light range is 190 – 390 (may vary depending on the books). The fluorescence of hydrocarbons like Salicylic acid can be observed by placing the TLC place under higher wavelength UV light that is 302 nm.
7. You have a mixture of salicylic acid, Naphthoic acid, and catechol. How will you differentiate them?
a) I will perform a TLC experiment using a polar solvent then will place the plate under higher wavelength UV light
b) I will perform a TLC experiment using a polar solvent
c) I will perform a TLC experiment using a non-polar solvent then will place the plate under higher wavelength UV light
d) I will perform a TLC experiment using a polar solvent then will place the plate under shorter wavelength UV light
View Answer
Explanation: Salicylic acid, Naphthoic acid and catechol all are hydrocarbons so the TLC experiment has to be performed using a non-polar solvent. Now, catechol will travel the lowest distance but salicylic acid and Naphthoic acid travel almost the same distance. Now to differentiate between salicylic acid and Naphthoic acid, we place placing the TLC place under higher wavelength UV light that is 302 nm, where salicylic acid will show fluorescence but Naphthoic acid will be non-fluorescent or less fluorescent.
8. Which of the two hydrocarbons gives almost the same Rf values in an analytical TLC plate?
a) Catechol and Naphthoic acid
b) Catechol and Salicylic acid
c) Catechol and Toluene
d) Salicylic acid and Naphthoic acid
View Answer
Explanation: Salicylic acid and Naphthoic acid gives almost the same Rf values in an analytical TLC plate as they move the almost the same distance. They can be further differentiated by higher wavelength UV exposure.
9. If the distance traveled by solute is 4 cm and distance traveled by Solvent is 6 cm, then what is the Rf value for that particular solute?
a) 0.6667
b) 1.5
c) 1
d) 2
View Answer
Explanation: Rf value = (Distance traveled by the solute molecule from the origin/ Distance traveled by the solvent front from the origin of samples). So, the Rf value is 4/6 = 0.6667.
10. What do you mean by Rf?
a) Distance traveled by solute from the bottom of the plate / Distance traveled by the solvent from the bottom of the plate
b) Distance traveled by solute from the origin / Distance traveled by the solvent from the origin
c) Distance traveled by solute from the bottom of the plate / Distance traveled by the solvent from the origin
d) Distance traveled by solute from the origin / Distance traveled by the solvent from the bottom of the plate
View Answer
Explanation: We compare the movement of the solute with the movement of the solvent. Therefore the travel distance both sample and solvent are measured from the sample spot origin not from bottom as below the origin, no sample was there.
11. Consider the following image as a TLC plate showing the movement of compound A and B. What is the ratio of Rf value of A and B?
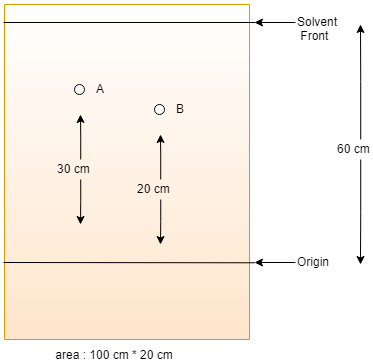
a) 2/6
b) 3/6
c) 2/3
d) 3/2
View Answer
Explanation: Rf value = (Distance traveled by the solute molecule from the origin/ Distance traveled by the solvent front from the origin of samples). So, the Rf value of A = 30/60 and the Rf value of B = 20/60, Therefore the ratio of Rf value of A and B will be 30/20 = 3/2.
12. Ninhydrin is used for the detection of _________
a) amino acids
b) fats
c) aromatic hydrocarbons
d) carbohydrate
View Answer
Explanation: Ninhydrin test is used for the detection of amino acids. The NH2 group of amino acid reacts with the Ninhydrin reagent to form a colored product.
13. The following image is a TLC plate made of a highly polar backbone (the static phase). Now arrange the following compounds named A, B, C, and D respectively in their decreasing order of polarity.
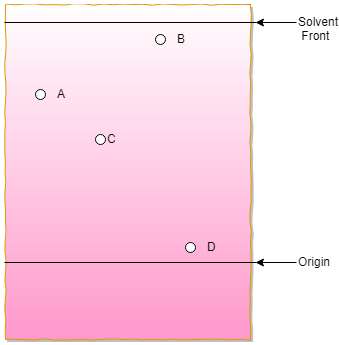
a) D>C>B>A
b) A>B>C>D
c) D>C>A>B
d) D>B>C>A
View Answer
Explanation: The backbone of the TLC plate is highly polar. So this static phage will highly attract to the polar compounds. So more the polar compound the sample is, more will be attracted to the polar plate, and it will travel a shorter distance. So according to this concept, the correct order is D>C>A>B.
14. Which of the following amino acid will have the highest Rf value in a non-polar solvent?
a) Glutamic acid
b) Histidine
c) Alanine
d) Leucine
View Answer
Explanation: Leucine is the most non-polar among the above options. So Leucine will travel the highest distance. Therefore, Leucine will have highest Rf.
15. Imagine 4 amino acids – A, B, C, and D. The Rf values for each amino acids are 0.33, 0.66, 0.44, 0.79 respectively. If the solvent was nonpolar and volatile then which one is the most polar amino acid and which is the most nonpolar amino acid?
a) The most polar amino acid is A and the most nonpolar amino acid is D
b) The most polar amino acid is D and the most nonpolar amino acid is A
c) The most polar amino acid is A and the most nonpolar amino acid is B
d) The most polar amino acid is A and the most nonpolar amino acid is C
View Answer
Explanation: With the increase in the non-polarity of the compound the sample will travel more distance. So according to this concept, Rf value for most nonpolar samples will be highest as Rf value is proportional to the distance traveled by the solute.
Sanfoundry Global Education & Learning Series – Biophysics.
To practice all areas of Biophysics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
