This set of Biophysics Multiple Choice Questions & Answers (MCQs) focuses on “Ion Exchange Chromatography – Set 2”.
1. Phosphate is the ionizable group of what type of ion exchangers for ion exchange chromatography?
a) DEAE-cellulose
b) CM-cellulose
c) DEAE-Sephadex
d) P-cellulose
View Answer
Explanation: P-cellulose contains phosphate group which can be ionizable by changing the pH of the medium. The other options doesn’t has any phosphate group in their molecular structure rather some are weakly basic.
2. What is the charge of proteins in cation exchange chromatography?
a) Negative
b) Positive
c) Neutral
d) Zwitterion
View Answer
Explanation: In cation exchange chromatography cation exchanged with the column. Therefore, the protein charge is positive and its gets attached with anionic column which is further eluted using concentrated NaCl solution.
3. CM cellulose is used as an anion exchange chromatography.
a) True
b) False
View Answer
Explanation: CM cellulose constitutes of weakly acidic molecules. Therefore, CM cellulose is used in cation exchange chromatography and acts as a cation exchanger.
4. In ion exchange chromatography, which of the following column has Carboxymethyl as its constituents?
a) DEAE-cellulose
b) DEAE-Sephadex
c) P-cellulose
d) CM-cellulose
View Answer
Explanation: Carboxymethyl or -CH2COOH is a part of CM-cellulose. CM cellulose is actually the short form of Carboxy-Methyl.
5. In cation exchange chromatography, the exchanger (column wall) is positive in charge.
a) True
b) False
View Answer
Explanation: In case of cation exchange chromatography, the cation is itself exchanged by the column wall. Therefore, the column wall must be negative in charge.
6. Chromatofocusing is a method separated protein on the basis of ___________
a) isoelectric points
b) charge of the molecule
c) molecular weight of the sample
d) viscosity of the sample
View Answer
Explanation: Chromatofocusing is a method separated protein on the basis of isoelectric points. In that case different poly cations and poly anions are used which make a gradient of pH. So proteins are separated on the basis of their pI.
7. Which of the following material (used in Ion Exchange Chromatography) is weakly basic?
a) DEAE-cellulose
b) CM-cellulose
c) P-cellulose
d) Bio-Rex 70
View Answer
Explanation: DEAE-cellulose is composed of -CH2CH2N(C2H5)2, which because of the presence of Nitrogen and two ethane groups, became positive in nature. Therefore this compound behaves as weak base.
8. For what purpose, we use resins in ion exchange chromatography?
a) As a cation Exchanger
b) As a supporting material
c) As a anion Exchanger
d) As a neutral exchanger
View Answer
Explanation: Resin is a non-reactive material. Thus rather than using it as an ion exchanger, resin is used as a supportive material.
9. What are the constituents of polyelectrolytes?
a) Cations
b) Anions
c) Both Cation and anions
d) Neutral ions
View Answer
Explanation: Polyelectrolytes are a mixture of cations and anions. These mixture of solution (polyelectrolytes) are used in chromatofocusing chromatography where proteins are separated on the basis of their isoelectric point.
10. You have 4 proteins P, Q, X, Y with overall charge 2+, 9+, 1-, 4+ respectively. Which protein will show the peak D in a cation exchange chromatography?
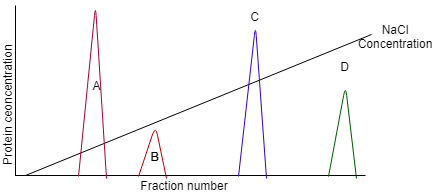
a) Protein P
b) Protein Q
c) Protein R
d) Protein Y
View Answer
Explanation: In a cation exchange chromatography, higher amount of NaCl is required for those proteins which has a higher overall positive charge on it. Thus, Protein Q (9+) will show the peak D.
11. The following image is the demonstration of cation exchange chromatography. Among the four proteins, one of the protein is negative. Identify the negatively charged protein.
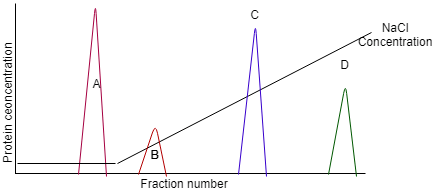
a) Protein A
b) Protein B
c) Protein C
d) Protein D
View Answer
Explanation: In a cation exchange chromatography, peak at higher NaCl concentration means higher positive charge it has. As Protein A doesn’t even need NaCl solution to be eluted that mean it was not previously bound to the column. Hence, protein A is negatively charged.
12. The following image is the conceptual demonstration of anion exchange chromatography. Identify the most positive protein in the following image.
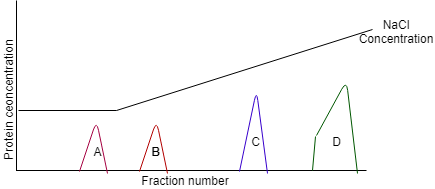
a) Protein A
b) Protein B
c) Protein C
d) Protein D
View Answer
Explanation: In an anion exchange chromatography, peak at higher NaCl concentration means the experimental protein has higher positive charge. In the above picture, protein A had been eluted at the lowest NaCl concentration, which suggests that the protein is positively charged.
13. You have four proteins with a different overall charge at pH 6. What technique you will perform to separate those proteins?
a) Adsorption chromatography
b) Ion Exchange chromatography
c) HPLC
d) Gel filtration Chromatography
View Answer
Explanation: Ion Exchange chromatography is the answer because the technique is based on the separation of the proteins on the basis their overall charge. Other chromatography techniques based on the size or adsorption.
14. Which of the following mixture cannot be separated using Ion Exchange Chromatography?
a) Mixture of cation and anions
b) Mixture of Cations
c) Mixture of two neutral and one anionic protein
d) Mixture of anions
View Answer
Explanation: In Ion Exchange Chromatography, proteins are separated on the basis of their overall charge. If two proteins have same charge or neutral at a particular pH (practically not possible) then they cannot be separated using Ion exchange chromatography.
15. If a molecule is highly positive, how can it be eluted using cation exchange chromatography?
a) Using elution buffer with higher concentration of KMO4
b) Using elution buffer with lower concentration of NaCl
c) Using elution buffer with lower concentration of KMO4
d) Using elution buffer with higher concentration of NaCl
View Answer
Explanation: If a molecule is highly positive, it can be eluted by using elution buffer with higher concentration of NaCl. Because a higher concentration of NaCl is required to exchange the protein from the column wall.
Sanfoundry Global Education & Learning Series – Biophysics.
To practice all areas of Biophysics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
